"Will Chantix really help me quit smoking?"
This article is no longer updated. It is archived for historical reference.
WARNING: If you or your loved one is using or considering using Chantix or Champix be sure and watch this safety warning video clip released by the U.S. Food and Drug Administration (FDA) on April 1, 2008, or read the warnings at the FDA website.
WARNING: Do not rely upon any information in this article to replace individual consultations with your doctor, pharmacist or other qualified health care provider.
"Does Chantix really work?" "Tell me the truth, what are my chances?" Frankly, not good. Putting aside FDA use risk concerns, if Chantix (whose chemical name is varenicline and sold as Champix outside the U.S.) is used as a stand-alone quitting aid without ongoing counseling or support, your chances of quitting smoking for one year are likely less than 1 in 11.
But if accompanied by 24 to 25 weekly counseling or support sessions of up to 10 minutes each, your chances could rise to 1 in 5 or even 1 in 4.
Yet sadly, Pfizer continues marketing Chantix as having a 44% success rate at 12 weeks, when that figure is really rather meaningless. Why? Because in clinical trials the treatment period was also 12 weeks. Chantix has a 24 hour elimination half-life and heavily counseled and supported users were still under its influence. If addicted to using an external chemical (nicotine) to satisfy a brain dopamine pathway "wanting" disorder (nicotine dependency), how significant is a boast about successfully substituting and using another chemical (Chantix) which satisfies your wanting disorder too?
Recent News
This article has been updated many times since Chantix's 2006 arrival. So, how do things stand with Chantix as the year 2014 arrives? There is still no long-term real-world population level study in which Chantix or any other approved quitting product defeats cold turkey quitters. None.
But there is evidence of cold turkey prevailing over quitting products on the market prior to Chantix arriving. And more recently, a July 2013 Gallup Poll found that only a tiny fraction of all successful ex-smokers (just 8 percent) succeeded by use of approved quitting products, including Chantix. As this new Gallup Poll screams, the pharmaceutical industry has been lying to smokers for decades about both their chances, and about how most quitters succeed.
As reviewed below, we have two studies in which Chantix failed to show statistical significance over nicotine patch in the percentage of successful long term quitters generated. We also saw evidence during 2011 suggesting that placebo-controlled Chantix studies have not been blind as claimed. But the most disturbing development during the past couple of years is a 2011 safety study whose conclusion actually discourages use of Chantix.
A November 2, 2011 study examined U.S. Food and Drug Administration (FDA) reports of serious adverse events in users of approved quit smoking products (Chantix, Zyban and NRT). It concluded:
"The findings for varenicline, combined with other problems with its safety profile, render it unsuitable for first-line use in smoking cessation."
Some of those "other problems" were shared in a May 19, 2011 report by the Institute for Safe Medication Practices (ISMP). According to that ISMP report:
- When examining U.S. adverse drug events reported to the FDA during the third quarter of 2010, varenicline accounted for "more possible cases than any other drug for suicidal/self-injurious behavior, depression, psychosis, hostility/aggression, and convulsions.
- Chantix ranks first in reported deaths, more than twice as many as any other drug regularly monitored by the ISMP.
- During the 3rd quarter of 2010, the FDA received 1,055 serious adverse drug event reports for Chantix, which again surpassed all other drugs regularly monitored by ISMP.
According to a July 6, 2011 ISMP email, through the 3rd quarter of 2010 the FDA had received a total of 36,342 U.S. reports of adverse events among varenicline users. Among them were "272 cases of completed suicide, 323 cases of suicide attempt and 63 cases described as suicidal behavior." ISMP noted previously identifying 408 cases of violence, which were defined as homicide, assault, physical abuse, violence-related symptom and homicidal ideation.
I shared some of the above ISMP findings in a July 7, 2011 Canadian Medical Association Journal (CMAJ) website online study response that I entitled "Varenicline's missing risk-benefit analysis." It was written in response to a varenicline cardiovascular risk article. There, I pointed out that we still have no proof that Chantix is effective as a stand-alone quitting aid when unaccompanied by formal counseling and/or support.
"What's it like using varenicline?"
On May 11, 2006, after completion of its drug approval package, the U.S. Food and Drug Administration (FDA) announced approval of the prescription drug varenicline, to be sold under the trade name Chantix. Interestingly, in the U.S. the FDA refused to approve Pfizer's use of the name "Champix" asserting that from a promotional perspective, "it is overly fanciful and overstates the efficacy of the product."
Today, Pfizer markets varenicline in all nations except the U.S. as Champix. Total global annual revenues during 2010 were $755 million (up from $700 million in 2009), with $330 million of those sales occurring in the U.S.
Still, for some, varenicline does have potential to both diminish wanting and decrease the rush sensed while still smoking during the first week of pre-quitting Chantix use. But, clearly there's a trade-off for those unable to avoid, move past or endure one or more of the nearly 200 potential side-effects listed on Pfizer's "Full Prescribing Information" sheet.
Prior to January 18, 2008. Pfizer's Patient Information sheet only mentioned vomiting, nausea, abnormal dreams, sleep disturbance and constipation as "the most common side effects." It failed to alert smokers to less frequent yet vastly more serious risks mentioned on varenicline's Full Prescribing Information Sheet, including suicidal thoughts, hallucinations, psychotic events, kidney failure, joint pain, muscle pain and arthritis.
Varenicline is a partial agonist that activates release of 35 to 60% of the dopamine that nicotine would have caused to flow if sitting on the exact same acetylcholine receptors. Being that varenicline is in pill form, if taken regularly it is present and occupying these receptors 24 hours a day.
In theory, within 4-5 days of taking Chantix and achieving "therapeutic levels," smoked bursts of nicotine arriving in the brain (which would normally generate a sense of relief from brain dopamine pathway wanting) should find most acetylcholine receptors already occupied.
One user described the expected yet missing wanting relief sensation as though "smoking a carrot." It's why Pfizer asserting that Chantix studies were "blind," that participants could not tell which group they had been randomized to (Chantix or placebo look-a-like sugar pills), is wishful thinking.
For many, it's not a matter of "guessing" whether or not a foreign chemical is present and at work inside their brain. A 2011 study suggests that possibly as many as 75 percent knew things were different, and they knew it before ever quitting smoking.
Although possibly less so than with nicotine patch, gum or lozenge use, Chantix quitters may experience some degree of back-end withdrawal syndrome upon ending varenicline use, as they attempt to re-adjust to natural dopamine pathway stimulation.
Pfizer, in its zeal to generate sales, continues to fail to adequately alert smokers and users to the rather important fact that half of clinical trial users who successfully used varenicline for 12 weeks, relapsed to smoking within a year.
While Pfizer does an outstanding job of explaining how varenicline occupies nicotine-type acetylcholine receptors, thereby blocking nicotine's ability to occupy the same receptor, it makes no attempt to explain what will happen should they inhale just one powerful puff of nicotine once Chantix/Champix is no longer present and blocking those receptors.
Pfizer marketing treats the topic of continued smoking while taking varenicline far too lightly (what it candy-coats as a "slip up"), thus setting the stage for relapse.
The message Pfizer should be pounding home is that after ending varenicline use, that just one puff and up to 50% of brain dopamine pathway receptors will become occupied by nicotine. While most walk away feeling like they've gotten away with "cheating," the mind's pay-attention pathways will soon make having done so nearly impossible, in the short term, to forget. Their entire Chantix experience will have been for naught, as their brain will soon be wanting or even begging for more nicotine.
Inflated success rates
Pfizer's five initial clinical trials of varenicline were published in July and August 2006. Three are comparable in that they involved a 12-week treatment period using 1mg of Chantix twice daily. In the Chantix study headed by Gonzales, 21.9% of Chantix users were still not smoking at one year. In Oncken the rate was 22.4% and in Jorenby 23%.
That's an average one-year rate of 22% or, on the flip side, a relapse rate of 78%. But these rates were achieved under highly artificial clinic study conditions. History and common sense teach that use under real-world conditions will likely generate a significantly higher failure rate. The question is, how high?
 Pfizer funded and co-authored the five initial studies and was involved in all study elements including design and monitoring. It spared no expense in creating what may be the most intense clinic quitting experiences ever.
Pfizer funded and co-authored the five initial studies and was involved in all study elements including design and monitoring. It spared no expense in creating what may be the most intense clinic quitting experiences ever.
Frankly, it's surprising that the intensity of support and interaction did not produce even higher rates. Real-world quitters, alone with their pills, or even participating in Pfizer's "GetQuit" support plan, will be fighting under entirely different battlefield conditions. What was it like inside an early Chantix study?
Users received their Chantix for free in all clinical trials. They were reimbursed travel expenses associated with clinic visits. They attended sixteen clinic visits involving brief one-on-one sessions with counselors trained in motivation and coping skills development. They received up to eight follow-up telephone support calls from their varenicline provider. In the earliest trials, they received two full physical exams, pondered the significance of a stream of questions in provider administered surveys, had their urine and blood checked seven times, sensed the seriousness associated with undergoing six EKGs, and watched their weight, vital signs and expired carbon monoxide breath tests recorded sixteen times.
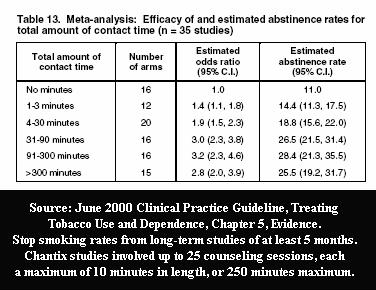 How much of Chantix's 22% one-year quitting rate is due to Chantix and how much attributable to the 26 times in the Jorenby study that participants spent up to ten minutes with their Chantix provider? How many real-world quitters will have the support benefit of 200 to 300 minutes with trained stop smoking counselors or their prescribing physician? Any? If so, at what financial cost? If not, at what cost in terms of performance?
How much of Chantix's 22% one-year quitting rate is due to Chantix and how much attributable to the 26 times in the Jorenby study that participants spent up to ten minutes with their Chantix provider? How many real-world quitters will have the support benefit of 200 to 300 minutes with trained stop smoking counselors or their prescribing physician? Any? If so, at what financial cost? If not, at what cost in terms of performance?
England's National Health Service (NHS) Stop Smoking Services (SSS) offers nationwide ongoing individual or group smoking cessation counseling and support that likely comes closest to the intensity and quality seen in Pfizer's studies. NHS also offers free varenicline (in the UK marketed as Champix). But NHS monitors and shares 4-week quitting rates, not 12-week rates like Pfizer. There's one other major difference. There are no placebo users in NHS SSS programs as placebo isn't a real quitting method.
According to 2010-11 UK NHS SSS nationwide data findings, four weeks after quitting 50 percent of non-medication quitters were still not smoking compared to 60 percent of varenicline users and 46 percent for NRT quitters (see 2011 see Table 4.1).
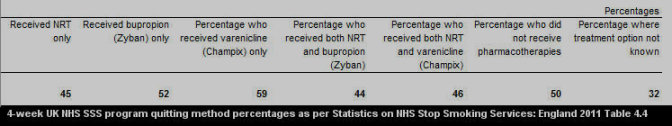
What's most notable about the above figures is that by the one month mark, non-medication quitters are already growing comfortable with natural dopamine pathway stimulation, while the average varenicline quitter still has another 8 weeks of treatment remaining before attempting to live without it.
While 50% of 2011 non-medication UK SSS quitters were still smoke-free at 4 weeks, in Pfizer's clinical trials only about 18% of placebo group quitters in the Jorenby study, 21% in the Oncken study and about 23% in the Gonzales study were smoke-free at 4 weeks. How can that be?
We know that varenicline's 12-week rate will decline by roughly half between weeks 12-52. History teaches that cold turkey quitters who are able to quit for a full month experience lower relapse rates than pharmacotherapy quitters at the same point, who have yet to end quitting product use. It's why conducting intellectually honest clinical trials which pit new products against real cold turkey quitters are so important. But with billions in profits at stake, it's why the industry cannot allow such studies to occur.
On some unknown date during 2007, Pfizer started offering Chantix users live telephone support as part of its new "GetQuit" program. Today, Pfizer's GetQuit.com website offers a counseling and support menu which includes "coaches," support email, phone calls, quitting tips, and immediate relapse crisis support if needed.
Truth be known, only a tiny fraction of millions of Chantix users have used the website. The vast majority appear to be using Chantix as a stand-alone quitting aid. According to December 30, 2011 website visitor traffic count data from Compete.com, the GetQuit.com website was then averaging only 5,176 visitors per day. By comparison, this site, WhyQuit.com, was then averaging 35,904 unique visitors each day. Keep in mind that Pfizer spends millions on Chantix marketing while WhyQuit doesn't spend a dime.
I reviewed a number of Pfizer's early GetQuit support emails and was generally in agreement with most user comments indicating that email support was rather lame. The only comments I've so far received regarding telephone support have been positive.
Early nicotine gum rates similar
Smokers who do not know the inflated and failed history of approved smoking cessation products are at greater risk of repeating it.
Chantix entered the quitting product market as a prescription aid at a time when nicotine replacement therapy or NRT was the clear front-runner. Nicotine gum was first approved by the FDA for prescription use in 1984 and was followed by the nicotine patch in 1991.
Both the gum and patch were approved for over-the-counter (OTC) sales in 1996, the same year prescription nicotine nasal spray was approved. The nicotine inhaler and bupropion (Zyban) joined as prescription products in 1997. In 2002 the lozenge become the first nicotine delivery device to enter the market directly as an OTC product.
We witnessed a feast to starvation difference between the intensity of support in randomized clinical trials compared to OTC NRT studies. The OTC studies were needed to validate the FDA allowing the nicotine gum and patch to go from prescription to OTC in '96. OTC study participants sometimes received little more than the instructions that came inside the box.
A 2002 study by pharmaceutical industry consultants combined and averaged the seven over-the-counter nicotine patch and gum studies. They found that just 7% were still not smoking at six months - a 93% relapse rate. Although a well-kept industry secret, OTC NRT's one-year rate is likely a bit less than 5%. Yes, a 95% failure rate and near 100% failure for second time users.
But NRT's extremely dismal quitting rate did not become visible until forced to stand on its own and be evaluated for OTC use. Until then, NRT was allowed to hide behind an intensity-rich clinic experience which nourished quitting motivations far longer than normal. Let's look back to a few of the more heralded early nicotine gum studies which often had heavy education, counseling and/or support elements too. How did their results compare to Chantix's initial 22% one-year rate?
Is Chantix/Champix a step forward or back? A 1976 nicotine gum study headed by Russell found that 23% of nicotine gum users were still not smoking at 1 year. The 1980 nicotine gum study by Raw produced a whopping 38% rate, in 1982 Jarvis found a 31% rate, in 1983 Schneider 30%, in 1984 Hialmarson 29%, in 1986 Daughton 31%, in 1987 Kornitzer 32%, and in 1989 Tonnesen boasted a 44% one year quit smoking rate.
The history of nicotine gum studies provided Pfizer confidence that intensively supportive Chantix (varenicline) studies should generate rather hefty and newsworthy one-year quitting rates. But it also knew that naked and alone, outside clinic doors, that nicotine gum proved at least three times less effective (using the low of 23% above to OTC NRT's 7% six-month rate).
Clearly, taking a Chantix pill twice daily is vastly easier than chewing piece after piece of nicotine gum, often after the onset of a crave episode. True, but aside from a spot check of the discouraging cost of a 12-week supply of Chantix (168 pills), which ranged from $506 to $624 (U.S.), Chantix users face the possibility of a lengthy list of discouraging side effects which, without counseling, explanation or ongoing support, may cause users to quickly abandon its use.
When used as a stand alone product, will Chantix's OTC quitting rate compare to OTC NRT's rate and be at least three times lower than the 22% average generated in Pfizer's three comparable studies? We don't yet know. Let's hope that the above, early one-year nicotine gum rates are not comparable as it could mean that Chantix's real-world rate might actually be worse than gum's.
When OTC NRT's dismal six-month 7% quitting rate is contrasted to the boasts within pharmaceutical industry NRT marketing commercials, clearly both smokers contemplating quitting and children contemplating smoking are being seriously deceived about the ease of quitting.
Pfizer could have turned a new page in placing honesty and openness above corporate profits. Instead, it has rewritten Aesop's tortoise and hare fable to suggest that a "hare" who wanted varenicline too but whose expectations were shattered by assignment to the placebo group, can be fairly compared to a cold turkey quitter who expected to endure and successfully navigate full-blown withdrawal. Pfizer knows that it cannot fairly pit its tortoise against real turkeys -- who want to quit cold turkey -- in open label/open method clinical trials without losing race after race, without being exposed and forfeiting billions in profits.
So what's the bottom line? What are your chances with Chantix or Champix? Clearly we don't yet know varenicline's odds when used as a stand-alone aid. If gum's performance is any indication we shouldn't be surprised to see one year rates in the neighborhood of 1 in 20 odds or 5%. But if able to fully duplicate the richness and intensity of support offered inside Pfizer's formal clinical trials, shouldn't users expect to experience 1 in 5 odds of success or 22% at one year? Although we wish it were true, maybe not.
Fails to defeat nicotine patch
Two varenicline versus nicotine patch clinical trials occurred after this article was written. They provide additional insight into varenicline's value as a real-world stand alone quitting aid (its effectiveness).
The first was a February 13, 2008 study by Aubin (link to free full text PDF copy) - This 52 week Pfizer funded study compared 12 weeks of varenicline (1mg twice daily) by 376 users, to 10 weeks of stepped-down nicotine patch use (21mg to 7mg patches) by 370 patch users.
As with Pfizer's five initial studies, the 2008 Aubin study didn't seek to demonstrate varenicline's worth under real-world conditions. Instead, it was designed to generate the highest one-year rate possible. The trial's artificial study conditions included excluding 21 percent of study applicants and intense counseling.
According to the Aubin study, "Counseling also occurred during every subsequent telephone and clinic visit." The study identified 1 baseline visit, 12 treatment phase visits for varenicline users (10 for patch users), with clinic visits on weeks 13, 16, 24, 32, 40, 48 and 52, interspersed with telephone counseling on day 3, and weeks 14, 20, 28, 36 and 44. That's at least 25 provider counseling sessions, each lasting up to 10 minutes in length.
The study measured cessation using two different methods, continuous abstinence and point prevalence. The study's continuous one year rate was 20.3% for nicotine patch users and 26.1% for varenicline users.
More alarming were the study's "7-day point prevalence of abstinence" rates. There, the study found that there ...
"were no significant differences" between Chantix and nicotine patch users at either 6 months (varenicline 38.6% vs. patch 34.1%) or one year (varenicline 34.8% vs. patch 31.4%).
As with all varenicline studies to date, this study provides zero evidence that any participant actually arrested their chemical dependency upon nicotine - none. Body fluids were not tested for either nicotine or cotinine, a longer lasting nicotine metabolite. In fact, according to the study, "use of NRT during the 9 months of follow-up did not disqualify a subject."
If Chantix use without 25 counseling sessions declines proportionally to known OTC nicotine patch rates, we're left with horrible six-moth rates.
Contrasting Aubin's one-year continuous abstinence rate with the earlier referenced 7 percent OTC NRT rate would proportionally put Chantix's six-month stand-alone rate at about 9% (7/20=9/26). The same analysis of its one-year point prevalence rate would suggest a slightly lower six-month rate of 8% (7/31.4%=8/38.6%).
Both 8% and 9% are lower than controls in U.S. Guideline evidence tables suggesting a natural six-month "on-your-own" quitting rate of 10-11%.
If accurate, the obvious question becomes, in conducting varenicline risk-benefit analysis, after factoring in escalating concerns over loss of life, why hasn't the FDA already ordered Chantix to be pulled from the market (see "FDA Chantix Handling Betrayed Public Health" for additional insights)?
The second patch versus Chantix study was published April 2010. It's primary author was Tsukahara (link to free full text PDF copy). A much smaller study than Aubin, the finding of the Tsukahara study was that:
"no significant difference in abstinence rates was observed between the 2 groups over weeks 9-12 and weeks 9-24."
Does it make sense to risk experiencing one of Chantix's rather serious side effects if it is no more effective long-term than using the nicotine patch?
28% of drug approval study applicants were denied participation
A second factor that could significantly diminish Chantix's real-world performance is associated with that fact that a substantial percentage of smokers who applied to participate in each study were excluded.
In Gonzales 1,843 smokers were screened and 458 were excluded (25%), in Oncken 980 were screened and 333 excluded (34%), and in Jorenby 1,413 were screened and 386 excluded (27%). But why?
A partial list of those excluded includes those suffering from cardiovascular disease, alcohol abuse, major depression, panic disorder, systolic blood pressure greater than 150 or diastolic pressure greater than 95, a history of cancer, a body mass index (calculated as weight in kilograms divided by height in meters squared) of less than 15 or higher than 38; weight less than 45kg, those with a "clinically significant medical disease," those over age 75 or younger than age 18, those smoking fewer than 10 cigarettes per day, and those known to have recently relapsed during NRT or Zyban quitting attempts. But why?
Most within these groups reflect populations that have historically been the most challenging to assist in quitting, including youth, who often smoke fewer than ten per day.
You'd think that government approval of any new medication would be conditioned upon all product marketing warning all groups excluded from the study that its safety or efficacy was not evaluated for them. Not so.
Clearly, Pfizer knew in advance that including difficult to treat populations, such as alcohol abusers, would have resulted in greater relapse rates, making final cessation figures less impressive (47% of relapses involve alcohol use - Brandon 1990).
Looking at the seriousness, types and quantity of adverse events now being reported (see safety discussion below), one has to wonder if some of the excluded groups, if included, would have revealed serious safety concerns sooner. If so, prior to excluding them, was Pfizer aware that excluding them would, to some degree, hide safety concerns? In other words, did Pfizer believe in advance that excluding the above groups would both elevate quitting rates and diminish safety concerns?
Pfizer's initial Chantix marketing aggressively assaulted all smokers, including most that it intentionally excluded from its studies. Visiting Pfizer's Chantix.com website on December 3, 2007, at first glance, it appeared to be inviting nearly all smokers to use it. The page then asked "if Chantix is right for me" but provides few answers. Instead, Pfizer was rather crafty in shifting the information burden to U.S. physicians in telling smokers to ask their doctor if Chantix is right for them.
Then, nearly all excluded groups had yet to be the focus of any serious study. Thus, at best, doctors could only guess as to how Chantix or Champix will interact with them. Six years later, a number of excluded groups still have little or no idea of their odds of success with Chantix or Champix, or their potential to experience adverse events.
Go to PubMed and search "varenicline" + key words associated with your condition or concern, to find out if it has yet been the focus of any study. Escalating concerns regarding varenicline side-effects are just now beginning to generate risk assessment papers for some excluded groups. But the number of quitters being evaluated and/or the haste with which these studies are being thrown together provides little confidence in relying upon their implications or conclusions.
For example, Pfizer's studies excluded those with mental health issues. We now have an August 2007 case study of one smoker diagnosed with bipolar disorder, whose condition was stable for five years while taking valproic acid. This man started experiencing manic symptoms within a week of taking 1mg of varenicline twice daily and had to be admitted to an inpatient psychiatric unit. Although noteworthy, one bipolar patient mixing valproic acid and varenicline does not a "study" make.
Pfizer has since scrambled to plug leaks in Chantix's use damn. Imagine trying to pass off old NRT and varenicline use data - data that included a brief medical history, with at least one question about mental health conditions - as a November 2007 safety study of varenicline use in patients with mental illness.
Imagine declaring varenicline use safe in patients with mental illness when: (1) only 53 patients asserting mental illness thereafter used varenicline; (2) none of the 53 underwent any objective mental health evaluation (see DSM-IV and ICD-10 standards) at any time during the study (nor did the control group); and (3) the study makes no reference whatsoever to any mental health medication being taken by any of the 53 patients.
What this junk "mental illness" study did find was greater depression in varenicline users. Also one user experienced a "severe psychological reaction likened to a bad LSD trip, including anxiety, paranoia, confusion and impaired motor control."
You'd think that nearly six years later, that Chantix clinical trial exclusion rates would have fallen below the average 28 percent rate seen in the original drug approval studies. Not so. A new exclusion record appears to have been set. Hawk 2011, a Chantix clinical trial study released November 30, 2011, evaluated extending pre-quitting Chantix use from the normal one week to four.
Participants in Hawk 2011 were a horrible reflection of real-world quitters. The study screened 359 applicants but only 60 were chosen. Talk about cherry-picking. That's an 83% non-participation rate, with just 1 in 6 participating.
Serious Chantix and Champix safety concerns
Since August 2006, when this article was written, varenicline safety concerns have continued to mount. Due to continuing developments this article's safety discussion is presented in chronological order. Each underlined date is a link to the development's online source.
May 10, 2006 - The FDA approves Chantix's Patient Information Sheet. The "Sheet" warns users of five "common side effects": nausea, changes in dreaming, constipation, gas, and vomiting. What it fails to alert patients to is the fact that varenicline's "Full Prescribing Information" sheet lists 160 additional potential adverse events. But as pointed out in a TV news interview with Dr. Ponni Subbiah, a Pfizer employee, all 165 "aren't necessarily associated with the drug, a causal association. It's just they were reported in the trials."
Additional Full Prescribing Information sheet adverse events originally listed included: (1) nightmares (2) headaches (3) dysgeusia a/k/a reduced sense of taste (4) somnolence a/k/a drowsiness (5) lethargy (6) fatigue/malaise/asthenia (7) rhinorrhea a/k/a runny nose (8) dyspnoea a/k/a difficulty breathing (9) upper respiratory tract disorder (10) rash (11) pruritis a/k/a itching (12) increased appetite (13) decreased appetite/anorexia (14) anemia (15) lymphadenopathy (16) luckocytosis (17) thrombocytopenia (18) splenomegaly a/k/a enlarged spleen (19) angina pectoris a/k/a chest pain (20) arrhythmia a/k/a irregular heart rate (21) bradycardia a/k/a heart rate less than 60 beats per minute (22) ventricular extrasystoles (23) myocardial infarction (24) palpitations (25) tachycardia (26) atrial fibrillation (27) cardiac flutter (28) coronary artery disease (29) cor pulmonale (30) acute coronary syndrome (31) tinnitus a/k/a ear ringing (32) vertigo (33) deafness (34) Meniere's disease (35) thyroid gland disorders (36) conjunctivitis (37) dry eye (38) eye irritation (39) vision blurred (40) visual disturbance (41) eye pain (42) acquired night blindness (43) blindness transient (44) cataract subcapsular (45 sleep disorder (46) ocular vascular disorder (47) photophobia (48) vitreous floaters (49) diarrhea (50) gingivitis (51) dysphagia (52) enterocolitis (53) eructation (54) gastritis (55) gastrointestinal hemorrhage (56) mouth ulceration (57) esophagitis (58) gastric ulcer (59) intestinal obstruction (60) acute pancreatitis (61) chest pain (62) influenza like illness (63) edema a/k/a swelling (64) thirst (65) chest discomfort (66) chills (67) pyrexia a/k/a fever (68) gall bladder disorder (69) hypersensitivity (70) drug hypersensitivity (71) liver function test abnormal (72) weight increased (73) electrocardiogram abnormal (74) muscle enzyme increased (75) urine analysis abnormal (76) diabetes mellitus (77) hyperlipidemia (78) hypokalemia (79) hyperkalemia (80) hypoglycemia (81) arthralgia a/k/a joint pain (82) back pain (83) muscle cramp (84) musculoskeletal pain (85) myalgia a/k/a muscle pain (86) arthritis (87) osteoporosis (88) myositis a/k/a muscle inflammation (89) disturbance in attention (90) dizziness (91) sensory disturbance (92) amnesia (93) migraine (94) parosmia (95) psychomotor hyperactivity (96) restless legs syndrome (97) syncope a/k/a fainting (98) tremor (99) balance disorder (100) cerebrovascular accident a/k/a stroke (101) convulsion (102) dysarthria a/k/a motor speech disorder (103) facial palsy a/k/a facial drooping (104) mental impairment (105) multiple sclerosis (106) nystagmus a/k/a involuntary eye movement (107) psychomotor skills impaired (108) transient ischemic attack a/k/a mini stroke (109) visual field defect (110) anxiety (111) depression (112) emotional disorder (113) irritability (114) restlessness (115) aggression (116) agitation (117) disorientation (118) dissociation (119) decreased libido a/k/a reduced sexual desire (120) mood swings (121) abnormal thinking (122) bradyphrenia a/k/a slowed thought processes (123) euphoric mood (124) hallucination (125) psychotic disorder (126) suicidal ideation a/k/a suicidal thoughts (127) polyuria a/k/a excessive volume of urination (128) nephrolithiasis a/k/a forming kidney stones (129) nocturia a/k/a waking during the night to urinate (130) urine abnormality (131) urethral syndrome (132) acute renal failure a/k/a kidney failure or injury (133) urinary retention (134) menstral disorder (135) erectile dysfunction (136) sexual dysfunction (137) epistaxis a/k/a nose bleeding (138) respiratory disorders (139) asthma (140) pleurisy (141) pulmonary embolism (142) hyperhidrosis (143) acne (144) dermatitis (145) dry skin (146) eczema (147) erythema (148) psoriasis (149) urticaria (150) photosensitivity reaction (151) hot flush (152) hypertension (153) hypotension (154) peripheral ischemia (155) thrombosis (156) abdominal pain (157) dyspepsia a/k/a indigestion (158) gastroesophageal reflux disease (159) dry mouth and (160) insomnia.
Both Pfizer on its Patient Information Sheet or the FDA at its website could have easily alerted smokers of the identity of health risk groups included within and excluded from clinical studies. Both chose to keep smokers in darkness.
Instead of only revealing the five most common side effects, the Patient Information Sheet could have told readers of the existence of 160 additional known risk concerns and directed them to either examine the Full Prescribing Information sheet or online FDA documents to see a complete list.
There, potential users would have learned the actual odds of experiencing the most common side effects. But again, only for the types of smokers included within the studies. They would have read that nausea was seen in 30% of users, insomnia in 18%, abnormal dreams in 13%, sleep disorder in 5%, nightmares in 1%, constipation in 8%, gas in 6% and vomiting in 5%.
They would also have noticed that Pfizer had classified all 165 potential side effects as either: (1) common; (2) frequent; (3) infrequent; or (4) rare. Although Pfizer's Chantix website directed smokers to ask their doctor, "Is CHANTIX (varenicline) right for me," the Full Prescribing Information sheet fails to provide physicians with the information needed to answer this critical question.
The sheet both fails to advise physicians of the identity of all groups Pfizer intentionally excluded from its five clinical trials, or define critical terms such as "frequent," "infrequent" and "rare."
Although the Prescribing sheet advised doctors that the five most common side effects (nausea, sleep disturbance, constipation, flatulence and vomiting) were experienced by greater than 5% of varenicline users, and that 19 other side effects listed as "common" occurred in greater than 1% of 1mg Chantix users, the terms "frequent," "infrequent" and "rare" are not defined.
All physicians are told is that these events reflect "a list of treatment-emergent adverse events reported by patients treated with CHANTIX during all clinical trials," which, the sheet indicates was "over 4,500 individuals."
It's as if Pfizer is toying with physicians, leaving clues here and there. For example, if your doctor had read the bottom of a September 18, 2007 Dallas Morning News story, Pfizer would have revealed to her or him that adverse events reported as "infrequent" occurred at a rate somewhere between 1 in 100 and 1 in 1,000 patients.
This would seem to suggest that "frequent" would be more often than in 1 in 100 patients and "rare" less than 1 in 1,000 patients. But if "frequent" is defined as events occurring more often that 1 in 100, where do "common" events fit into the puzzle?
Physicians attempting to analyze and properly advise patients regarding varenicline's risk puzzle are clearly left guessing as to how often adverse events should be expected, which among the 165 listed are actually caused by Chantix or Champix, and how their patient's chronic medical condition, and medications prescribed to treat it, will mesh with varenicline use.
October 4, 2006 - On this date a Chantix user named Suzy posted to a message board at WrongDiagnosis.com complaining of significant joint and muscle pain which started three days after she started taking Chantix and continued long after its use ended. Suzy closed by asking, "has anyone else experienced this?" As of December 30, 2011 Suzy had received 2,532 replies, many documenting Chantix muscle and joint pain nightmares significantly worse than hers.
June 1, 2007 - On this date a physician named Antonio Howell, MD began replying to Chantix user comments to his blog, a blog in which he listed the psychiatric disorders mentioned on Chantix's Full Prescribing Information sheet: "Frequent: Anxiety, Depression, Emotional disorder, Irritability, Restlessness. Infrequent: Aggression, Agitation, Disorientation, Dissociation, Libido decreased, Mood swings, Thinking abnormal. Rare: Bradyphrenia, Euphoric mood, Hallucination, Psychotic disorder, Suicidal ideation."
On June 1, 2007, Deanna told Dr. Howell how her husband had never had any mental health problems but tried to take his own life after being on Chantix for 13 days. On August 27, 2007 Zezrie wrote Dr. Howell telling him how her brother-in-law shot and killed himself while on Chantix. Since then Dr. Howell has been overwhelmed by additional mental health horror stories. He recently started a poll asking visitors if Chantix should be taken off the market until additional safety studies are done. As of December 2, 2007, 70% responding said "yes" (38 of 56).
September 18, 2007 - A Dallas Morning News story reported on the Labor Day death of Carter Albrecht, a local musician who mixed heavy alcohol use (3 times the Texas legal driving limit, a fact first reported on October 22, 2007) with Chantix use, became delusional, aggressive, assaulted his girlfriend, and minutes later was "shot and killed breaking into a neighbor's house."
A statement by Pfizer attached to the bottom of the Carter Albrecht story seems to blame his death on the act of quitting, not varenicline. Pfizer asserts, "It is important to note that a vast body of medical literature has shown that smoking cessation, with or without treatment, is associated with nicotine withdrawal symptoms and has also been associated with the exacerbation of underlying psychiatric illnesses."
Before accepting Pfizer's contention (which the FDA now appears to be buying into hook, line and sinker) we'd be wise to ask ourselves, with more than 46.5 million Americans having already successfully quit smoking, and CDC data indicating that almost 90% quit cold turkey, how many news stories have any of us ever read in which quitting cold turkey (without using any pharmacology product) was blamed for someone committing suicide, hallucinating, developing a psychotic disorder, horrible muscle or joint pain, or during which the quitter vomited for twelve straight weeks? Any?
September 24, 2007 - CBS 11 News in Dallas/Fort Worth broadcasts a story entitled "Miracle Drug or Dangerous Problem" (click link to watch the video clip). It reports on Carter Albrecht's death. It also pictures Karen from Maryland and Deborah in Oregon who both felt suicidal while using Chantix, and Candace in Arizona who experienced aggression. It interviews Scott Mullins who experienced bad dreams and horrible thoughts. "As much as I hate to admit it," says Scott, "there have been times that I thought about ending my life."
September 2007 - Public Citizen, a nonprofit, nonpartisan group which champions U.S. consumer interest, placed varenicline on its worst pills list, recommending that consumers not use it until 2014. Public Citizen cites varenicline's common side-effects being reported by more than one-third of clinical trial users and contends that safety information is currently inadequate.
October 25, 2007 - CBS 11 News in Dallas broadcasts a second story entitled "Drugs Tested on Few Before Released to Masses." It questions how Chantix could be approved for use after testing upon only 4,500 smokers. In it, CBS 11 News claims to have accessed the U.S. Food and Drug Administration (FDA) side effects data base and found "thousands of similar and very serious reactions to Chantix."
It notes that Pfizer tested varenicline on roughly 5,000 users prior to FDA approval and although its original physician "Full Prescribing Information" sheet warned doctors of a potential risk of "suicidal ideation," "aggression" and "nervous system disorders" that the only risks mentioned on Pfizer's original Patient Information sheet were the five most common side effects.
The CBS 11 story asserted that new signals had caused the European Commission on Human Medicines to put Champix on its list of "New Drugs Under Intensive Surveillance" and that it was now keeping a close eye on potential "suicidal thoughts and behaviour" in European patients prescribed Champix.
October 25, 2007 - Omer Jama, a popular 39 year-old UK television editor is found dead with his wrists slashed at his Bolton, England home, four weeks after starting Champix. According to his brother, "He's got no history of depression and was never the sort of person you would see feeling sorry for himself."
November 20, 2007 - The FDA announces that it "has received reports of suicidal thoughts and aggressive and erratic behavior in patients who have taken Chantix." It reports that "preliminary assessment reveals that many of the cases reflect new-onset of depressed mood, suicidal ideation, and changes in emotion and behavior within days to weeks of initiating Chantix treatment" and that as soon as its "analysis is completed, FDA will communicate its conclusions and recommendations to the public."
My question regarding the FDA's 11/20/07 announcement is who actually wrote it, the FDA or Pfizer, and whose interests are being protected, Pfizer's or the consumer's? Why does the announcement fail to share the gravity of the situation by at minimum revealing the total number of suicides among U.S. Chantix users that have thus far been reported to the FDA? Is this fact a national secret?
Compare the actual language from a statement Pfizer had the Dallas Morning News attach to its Chantix suicide story on September 18, 2007, to the actual language contained in the FDA's investigation announcement that was written 2 months and 2 days later. Then ask yourself, who authored the FDA announcement?
- 09/18/07 Pfizer Statement - "... smoking cessation, with or without treatment, is associated with nicotine withdrawal symptoms and has also been associated with the exacerbation of underlying psychiatric illnesses"
- 11/20/07 FDA Announcement - "... smoking cessation, with or without treatment, is associated with nicotine withdrawal symptoms and has also been associated with the exacerbation of underlying psychiatric illness."
According to a November 26, 2007 Chantix article, the Prescription Drug User Fee Act is a 1992 law that allows the FDA to bill pharmaceutical companies for the cost of approving their products, with the FDA estimating 2007 collections under the Act exceeding $259 million.
The article shares the insights of Dr. Sidney Wolfe, director of health research for Public Citizen. Dr. Wolfe indicates that the Act has resulted in a cultural shift at the FDA with quicker turnaround times and a more accommodating attitude toward drug makers. "The (pharmaceutical) industry is viewed by some people in the FDA as 'our client.' 'They're paying our salaries.' Twenty years ago, if a drug went through clinical trials and there were more serious questions, the attitude was, 'Let's do more studies.'" According to Dr. Wolfe, "'Not anymore.'"
November 28, 2007 - A Texas television station announces it used the Freedom of Information Act to obtain "a computer disc ... with 5,157 complaints, which were all filed in just one week after the News 8 report aired." This report asserts that, "suicide was reported 55 times," suicidal thoughts were mentioned in 199 cases, 417 people complained of depression and there were hundreds of mentions of anger, aggression, amnesia, hallucination and homicidal thoughts.
December 10, 2007 - Australia's Therapeutic Goods Administration (TGA) announces that it has reviewed reports submitted to the US FDA and ordered Pfizer to issue "Dear Doctor" letters and amend the Champix product information sheet. The warning states, "there have been reports of depressed mood, agitation, changes in behaviour, suicidal ideation and suicide in patients attempting to quit smoking while taking Champix." Sale of Champix is scheduled to commence in Australia on January 1, 2008.
December 14, 2007 - The European Medicines Agency (EMEA) announced that "updated warnings to doctors and patients are needed to increase awareness of cases of suicidal ideation and suicide attempts reported in patients using Champix (varenicline)."
January 17, 2008 - Pfizer announced that it had "updated the Chantix label in the U.S. to include a warning that patients who are attempting to quit smoking with Chantix should be observed for serious neuropsychiatric symptoms, including changes in behavior, agitation, depressed mood, suicidal ideation and suicidal behavior."
January 18, 2008 - Pfizer updated the safety information section of its Chantix website to warn visitors that, "You should be aware that some patients have reported depressed mood, agitation, changes in behavior, suicidal thinking or behavior when attempting to quit smoking while taking CHANTIX. If you experience any of these symptoms, or if your family or caregiver observes these symptoms, please tell your doctor immediately." "If you have ever had depression or other mental health problems, tell your doctor before taking CHANTIX. Quitting smoking, with or without CHANTIX, can result in nicotine withdrawal symptoms (such as depressed mood, agitation) or a worsening of underlying psychiatric illness, such as depression."
January 18, 2008 - Pfizer revised the Patient Information sheet to include an extremely weak and watered-down warning that suggests that all quitters, including Chantix quitters, may experience suicidal thoughts. It reads, "Tell your doctor if you experience agitation, depressed mood or suicidal thoughts. These symptoms have been reported in patients trying to stop smoking with or without Chantix. It is not known if these symptoms are related to Chantix."
Shockingly, the Patient Information sheet keeps hidden what Pfizer reveals to those having Internet access, to those visiting its Chantix website, that we are not just talking about suicidal "thoughts" but suicidal "behavior." Also, Pfizer continues to suggest that thinking about killing yourself is a normal and expected risk factor for cold turkey quitters too. We have 48 million comfortable ex-smokers in America. Where are the news stories sharing details about how cold turkey quitting produces risk of suicidal thoughts or behavior?
We have more than 200 nicotine replacement therapy (NRT) studies that followed tens of thousands of quitters, that detail the side effects and risks experienced by each group within each study? Where are the NRT studies mentioning suicidal thoughts, suicide, aggressive behavior or psychotic events?
January 18, 2008 - Pfizer updated its Full Prescribing Information sheet to warn physicians about "Neuropsychiatric Symptoms." The warning reads, "Serious neuropsychiatric symptoms have occurred in patients being treated with CHANTIX. Some cases may have been complicated by the symptoms of nicotine withdrawal in patients who stopped smoking; however, some of these symptoms have occurred in patients who continued to smoke. All patients being treated with CHANTIX should be observed for neuropsychiatric symptoms including changes in behavior, agitation, depressed mood, suicidal ideation and suicidal behavior. These symptoms, as well as worsening of pre-existing psychiatric illness, have been reported in patients attempting to quit smoking while taking CHANTIX in the post-marketing experience. Patients with serious psychiatric illness such as schizophrenia, bipolar disorder, and major depressive disorder did not participate in the pre-marketing studies of CHANTIX and the safety and efficacy of CHANTIX in such patients has not been established. Patients attempting to quit smoking with CHANTIX and their families and caregivers should be alerted about the need to monitor for these symptoms and to report such symptoms immediately to the patient's healthcare provider."
While Pfizer at last reveals to healthcare providers that varenicline use was never studied in psychiatric patients, it continues to keep them in darkness as to all other classes of patients who were excluded from clinical trials, for which varenicline risks were not studied and are still unknown, including all with clinically significant medical conditions and all abusing alcohol.
February 1, 2008 - The FDA announces "important revisions to the WARNINGS and PRECAUTIONS sections of the prescribing information for Chantix regarding serious neuropsychiatric symptoms experienced in patients taking Chantix. These symptoms include changes in behavior, agitation, depressed mood, suicidal ideation, and attempted and completed suicide."
The FDA's February 1, 2008 "Public Health Advisory" goes to the extreme of enlisting families of varenicline users to remain "alert to and monitor for changes in mood and behavior in patients treated with Chantix. Symptoms may include anxiety, nervousness, tension, depressed mood, unusual behaviors and thinking about or attempting suicide. In most cases, neuropsychiatric symptoms developed during Chantix treatment, but in others, symptoms developed following withdrawal of varenicline therapy."
February 10, 2008 - New York Magazine publishes "This is My Brain on Chantix," a firsthand Chantix use account by Derek de Koff, a features writer, who after taking Chantix experienced vivid dreams, blackouts, hallucinations and contemplated suicide.
April 1, 2008 - The FDA release a new two and a half minute Chantix safety warning video clip that for the first time admits "links" to serious neuropsychiatric problems in users, including suicide.
April 2, 2008 - WhyQuit article expresses concerns over FDA video clip not advising users of the actual number of suicides and not sharing honest efficacy and effectiveness info needed to make informed use decisions.
April 3, 2008 - FDA pulls video clip. WhyQuit makes video pulled clip available for viewing. Public Citizen calls upon the FDA to issue a "black box" Chantix warning, a warning reserved for drugs linked to serious or life-threatening adverse events, the strongest warning the FDA can mandate.
May 7, 2008 - USHHS released updated tobacco treatment Guideline giving Chantix equal recommendation weight with NRT and Zyban. Interestingly, the Guideline recommends use of Chantix on PDF pages 5, 7, 25, 60 and 62 but waits until page 63 to first mention its association with suicide. The 25 member private-sector panel authoring the Guideline had significant pharmaceutical industry financial ties.
May 16, 2008 - The FDA announced a name change and updating of the "Patient Information" sheet, which is now referred to as a "Medication Guide" and updating of the "Prescribing (labeling) Information" sheet, with an updated "Information for Healthcare Professionals" page. The Medication Guide moves patient notice regarding risks of changes in behavior, agitation, depressed mood, and suicidal thoughts or actions from the tail end of the 01/18/08 version to the front of the revised guide. But in the paragraph following the warning the FDA permits Pfizer to use the normal, temporary sense of emotional loss accompanying all cessation attempts, to water down the above warning by asserting, "When you try to quit smoking, with or without CHANTIX, you may have symptoms ... including ... depressed mood ... irritability ... anger ..." The revised Prescribing sheet tells providers to advise patients to not only report behavioral symptoms but immediately stop taking Chantix.
May 21, 2008 - A media firestorm hits Chantix as an ISMP study is released examining 6,363 U.S. Food and Drug Administration adverse drug reaction reports implicating Pfizer's quit smoking pill Chantix, and 3,063 are found to involve serious injuries, including 78 deaths, only 28 of which were from suicide. FDA is criticized for only focusing almost exclusively on behavioral death risks when numerous reports suggest cardiac causes, both thromboembolic and arrhythmic. It states that by the end of 2007 "varenicline accounted for more reports of serious drug adverse events in the United States than any other drug." The study recommends in part that smokers "consider the use of alternative approaches to smoking cessation."
May 21, 2008 - The Federal Aviation Administration banned pilots and air traffic controllers from using Chantix based upon the above ISMP study implicating Chantix in contributing to 173 serious accidental injuries.
May 22, 2008 - Impacting truckers and bus drivers, the Federal Motor Carrier Safety Administration announced that, "medical examiners should not certify a driver taking Chantix because the medication may adversely affect the driver's ability to safely operate a commercial motor vehicle."
May 29, 2008 - Pfizer goes into damage control mode and purchases full-page Chantix ads in America's 5 largest newspapers, including this ad from the New York Times.
July 1, 2009 - The FDA issues a new public health advisory notifying the public that it is requiring the manufacturers of both Chantix and Zyban (bupropion) to add a new Boxed Warning to the product labeling to alert healthcare professionals to risks of hostility, agitation, depressed mood, and suicidal thoughts or actions. Listen to an FDA Podcast of the event or read the FDA's press release.
August 19, 2009 - Saudi Arabia Ministry of Health bans Champix as causing serious side effects including suicide.
October 1, 2009 - A study in the British Medical Journal (BMJ) reviews data on nearly 11,000 British varenicline users and finds that although a twofold increased risk of self harm with varenicline cannot be ruled out, there was no evidence that varenicline was associated with an increased risk of depression (n=2,244) or suicidal thoughts (n=37).
October 18, 2009 - The senior scientist for the Institute For Safe Medication Practices and a Wake Forest School of Medicine professor respond to the BMJ article sharing contrary UK data showing 377 cases of suicidal thoughts, 46 attempted suicides and 22 completed suicides among 5,110 UK varenicline users.
November 4, 2009 - The authors of the BMJ article reply defending their article. They close by advising that doctors prescribe varenicline with caution, that patients should be told to stop treatment and contact their doctor immediately if they develop suicidal thoughts or behavior, that varenicline should be stopped immediately if agitation, depressed mood, or changes in behavior are observed that are of concern to the patient, family, or caregivers, and that the safety and efficacy of varenicline in people with serious psychiatric illness have not been established. Patients who have a history of psychiatric illness should be monitored closely while taking varenicline.
December 29, 2009 - Pfizer purchases full page ads in U.S. newspapers in anticipation of New Year's, the biggest quitting day of the year. The theme? "I honestly loved smoking. And I honestly didn't think I would ever quit." "With Chantix you can smoke during the first week of treatment."
July 20, 2010 - A study published in Annals of Pharmacotherapy examined selected Chantix user FDA adverse event reports that included 10 assault, 9 cases of homicidal ideation, and 7 cases of other thoughts or acts of aggression/violence. It concluded that, "The clear temporal relationship, lack of prior history of this behavior, and unusual nature of these events strengthens the accumulating scientific evidence that varenicline is associated with thoughts and acts of aggression/violence. We recommend that physicians and pharmacists ensure that all patients are informed of possible psychiatric symptoms of varenicline, including violent and aggressive thoughts. All patients should be advised to contact a health-care provider immediately if these symptoms occur and varenicline should be discontinued without delay.
December 15, 2010 - A study published in PLOS examined violence towards others reported as medication adverse events to the FDA. Varenicline ranked highest in proportional reporting among the 31 drugs for which violence was reported.
January 5, 2011 - A University of Newcastle professor's letter in the Journal Addiction questions whether or not Pfizer's controversial quit smoking pill varenicline is worth it. Also, the latest English varenicline quitting rate data suggests that Pfizer is vastly overstating the odds of success that real-world quitters should expect.
May 19, 2011 - The Institute for Safe Medication Practices (ISMP) issues a report indicating that the number of U.S. suicides blamed on Chantix has more than doubled from 122 to 272. During the 4th quarter of 2010, the FDA received 1,055 serious adverse drug event reports for Chantix. The number of reported deaths blamed on Chantix remains twice that of any other monitored drug. In response to an email question the ISMP states that the total number of U.S. reported adverse drug events of all severity now exceed 35,000, with roughly 10,000 of those events being serious, disabling or fatal.
June 1, 2011 - France bans Champix reimbursement because of questions about its safety.
June 7, 2011 - While Pfizer Chantix marketing continues to boasts a 44% success rate, a new clinical study finds that only 1 in 7 Chantix quitters were still not smoking at 6 months.
June 16, 2011 - FDA issues a safety announcement that varenicline may be associated with a small, increased risk of certain cardiovascular adverse events in patients who have cardiovascular disease, including heart attack.
November 2, 2011 - A new FDA adverse events study entitled "Suicidal Behavior and Depression in Smoking Cessation Treatments" identified "3,249 reported cases of suicidal/self-injurious behavior or depression, 2,925 (90%) for varenicline, 229 (7%) for bupropion, and 95 (3%) for nicotine replacement."
It concluded that, "The findings for varenicline, combined with other problems with its safety profile, render it unsuitable for first-line use in smoking cessation."
November 24, 2011 - The FDA issues a "Safety Announcement" that although new hospitalization study of risk of neuropsychiatric adverse events found no difference between Chantix and NRT, that the study does "not rule out an increased risk of other neuropsychiatric events with Chantix." For example, obviously, a person successful at suicide is not normally hospitalized.
December 12, 2012 - The FDA issues a "Safety Communication" on Chantix cardiovascular risks stating that "a higher occurrence of major adverse cardiovascular events (a combined outcome of cardiovascular-related death, nonfatal heart attack, and nonfatal stroke) was observed in patients using Chantix compared to placebo."
July 31, 2013 - After 7 years on the market, Chantix fails to receive specific mention in a Gallup Poll asking ex-smokers how they succeeded in quitting, with all prescription quitting products combined being credited with only 2% of successful cessation.
August 8, 2013 - Surveys sent to 6,882 women of reproductive age found that 19 had been exposed to varenicline during pregnancy, with exposure ranging from 1 day to 16 weeks. "Adverse outcomes were identified in five of 17 live births: one baby had birth asphyxia and recurrent chest infections, one had gastro-oesophageal reflux, one was diagnosed with ankyloglossia and two had feeding difficulties."
August 14, 2013 - Researchers in India, with a smokeless tobacco use rate of 20%, conducted a placebo controlled trial of varenicline among 237 smokeless tobacco users. At end of treatment (12 weeks) "abstinence was greater for varenicline versus placebo (25.2% vs. 19.5%), but this was not statistically different."
October 11, 2013 - A large U.K. study of 120,000 quitters published in the British Medical Journal finds "no evidence of an increased risk of suicidal behaviour in patients prescribed varenicline or bupropion compared with those prescribed nicotine replacement therapy."
October 28, 2013 - A UK psychiatrist professor responds to the new UK BMJ study asserting that "the confidence intervals for both suicide and self-harm are too wide to conclude that there is no link with suicidal behaviour," that "while clearly these drugs are helpful to many who want to stop smoking, we can not on the basis of this study withdraw cautionary advice that some individuals may experience significant psychiatric side effects from them."
Cessation pharmacology history has never before seen the frequency and severity of the adverse events now being attributed to Chantix and Champix, with many lingering long after use ends, some permanent or fatal. If varenicline's "real-world" benefits (if any) do not significantly exceed the degree of risk use poses, times the probability of those risks occurring, it should be pulled from the market.
Frankly, today, no government can tell us if varenicline's benefits exceed its risks. Our current drug approval process is upside down. Real-world use conditions studies that could have answered the risk-benefit question were not conducted prior to approving varenicline's sale, and have not been undertaken since. Instead, health officials place industry profit concerns instead of consumer safety and product effectiveness.
If Pfizer knows the actual odds of experiencing any "rare" yet significant side effect, does it have an obligation to share the actual odds with users? In regard to "frequent" and "infrequent" side effects, if Pfizer does not know the actual odds of experiencing those it has listed, should it?
Are Chantix and Champix users, and their physicians, entitled to the relative-risk information needed in order to make informed and intelligent cessation product use decisions?
How to report adverse reactions to Chantix or Champix
All Chantix or Champix users experiencing significant adverse events are strongly encouraged to report them to your government's adverse event reporting agency. Varenicline is a relatively new drug and without adequate user feedback medication safety officials may remain in relative darkness regarding some risks for years or even decades. The following are links to online reporting forms: Australia | Canada | New Zealand | United Kingdom | United States
Pfizer Giving Refunds
I am still receiving reports of Pfizer giving purchase price refunds to users experiencing reactions and unable to continue taking varenicline. Rachael from Tennessee had used Chantix for 5 days before developing a rash on her face. If you live outside the U.S. ask your local pharmacist how to request a refund. If you live in the U.S. here's how to get your money back.
I most recently telephoned Pfizer's toll-free automated U.S. Refund Request Line on June 9, 2011 at 1-800-220-9496 to verify the refund process. According to the Refund Request Line, patients experiencing adverse reactions need two things to make a refund request: (1) a letter signed by you stating the reason for your refund request; and (2) a copy of your pharmacy purchase receipt for your last filled prescription that contains your patient name, pharmacy contact information, prescription number, drug name and quantity, your contact info, the cost to you, and the date filled. If you no longer have your receipt Pfizer suggests obtaining a duplicate from your pharmacy.
Mail your short letter and receipt to:
Pfizer Product Refund Program
6501 Weston Pkwy, Suit #370
Cary, N.C. 27513
Pfizer indicates that it generally takes 2 to 4 weeks to process a refund request. Be sure and make a copy of both your receipt and letter prior to sending it. If you have any trouble you can talk to a human by calling Pfizer Customer Service at 1-800-438-1985, and while you have their attention be sure to have them document any and all side effects you experienced while using it. You can also visit Pfizer's Customer Service Page.
Clinical trials not blind as claimed
It's hard to imagine any smoker who has not heard the NRT marketing assertion that it "doubles" your chances of quitting. If Pfizer and Chantix follow the path taken in marketing NRT, regardless of how low Chantix's quitting rate eventually becomes, smokers will only be told its victory margin over the study's placebo group, not the actual percentage of users succeeding.
In fact, it's already happened. Pfizer's May 11, 2006 Chantix press release failed to disclose that nearly 4 out of 5 Chantix clinical study participants relapsed to smoking. Instead it boasts, "In two identically designed studies, patients receiving a 12-week course of Chantix therapy (1 mg twice daily) nearly quadrupled the likelihood of quitting than those taking placebo."
Let's reflect on these massive Chantix placebo victories. In Chantix clinical trials, those screened and not excluded were randomly assigned to Chantix (the active group), to another quitting product if being compared (Zyban or NRT), or to the study's placebo control group, a group which would normally receive an inert sugar pill that otherwise looks identical to varenicline.
Each of the five Chantix studies represents that it was double blind. If true, neither participants nor researchers should have been able to determine participant assignment to either a placebo pill or the active chemical varenicline. Surprisingly, drug approval studies do not mention whether or not researchers actually conducted blinding integrity assessments to test and validate the study's blind.
Blinding is extremely important to the study's core validity. If randomized participants can determine their group assignment then the study's final odds ratio victory (if any) may reflect frustrated and/or fulfilled expectations rather then the actual merits of the chemical tested.
While placebo controls remain the gold standard in most research areas, smoking cessation treatments are unique in seeking to minimize a condition - withdrawal - which does not exist until its onset is commanded by researchers ("ready, set, quit"). It may be the only study area where participants randomized to placebo are actually punished with significant withdrawal anxieties and made significantly more distressed than when they arrived.
We've known since June 2004 that NRT studies were generally not blind as claimed. On November 30, 2011 we were shown evidence that Chantix studies were not blind either.
Nicotine is a psychoactive chemical, a central nervous system and brain dopamine pathway stimulant. The pharmaceutical industry has known since at least a 1994 study that smokers can quickly be trained to reliably distinguish various doses of nicotine from placebo . Smokers with a prior quitting history have experienced their own withdrawal syndrome and should be expected to recognize both its onset and intensity.
Chantix studies report that varenicline significantly diminishes a smoker's withdrawal syndrome. Cravings were consistently reduced when varenicline, 1.0 mg twice daily, was compared with placebo. All three comparable studies found that varenicline significantly reduced the urge to smoke compared to placebo.
Approximately 90% of participants in Chantix's early drug approval studies had previously attempted quitting, failed and had some degree of memory of what it felt like to sense the onset of the anxieties and craves associated with their withdrawal syndrome.
A June 2004 study by Mooney reviewed 73 allegedly double-blind NRT studies and declared that the limited number of studies assessing blindness were not generally blind as claimed in that "subjects accurately judged treatment assignment at a rate significantly above chance."
"To determine the prevalence of failure, clinical trials of NRT should uniformly test the integrity of study blinds," Mooney asserted. "Moreover, if blindness failure is observed, subsequent efforts should be made to determine if blindness failure is related to study outcome and, if so, to provide an estimate of treatment outcome adjusted for blindness bias. Without these methods and analyses, the validity of NRT clinical trial results could be questioned."
The blinding analysis in a 2005 study by Dar found that 3.3 times as many placebo group members correctly guessed that they had received placebo (54.5%) as guess nicotine (16.4%). Although the Dar study focused on smoking reduction, Tonnesen's 1993 nicotine inhaler quitting study produced strikingly similar placebo group findings in that 3.8 times as many in the placebo group correctly guessed placebo (58%) as guessed nicotine (15%). Among inhaler users, Tonnesen found that 3.5 times more correctly guessed inhaler (46%) as guessed placebo (13%), while 42% on active and 27% on placebo did not know which treatment they had received.
More recently, a June 2009 study by the nicotine patch co-inventor, Dr. Jed E. Rose, found that "of 165 subjects receiving placebo patches, 27 believed they had received active patches, 112 believed they had not, and 26 were unsure." Yes, four times as many placebo group members correctly identified their assignment as could not.
Participants were recruited to Chantix studies by being told that the study involved evaluation of a medication. Most seeking participation knew their withdrawal syndrome and clearly hoped the medication would diminish it.
Pfizer knew that NRT studies were plagued by blinding failures and that frustrated and rewarded expectations likely played a substantial role in both relapse and cessation. Pfizer also knew that Chantix placebo group members would not be receiving anything different than received by NRT placebo group members - an inert placebo.
For them, the exact same set of elements was in play: the expectation of relief; the onset of full withdrawal; and frustrated expectations. It knew that the active group would sense a "significant" reduction in their withdrawal syndrome and thus likely be more inclined to remain and take advantage of the study's heavy and lengthy counseling and support structure.
Mooney's warning that a study's core validity could be questioned for failure to test the study's blind went unheeded by any Chantix study until November 30, 2011 and the publication of a paper by LW Hawk, et al entitled, The Effects of Extended Pre-Quit Varenicline Treatment on Smoking Behavior and Short-Term Abstinence: A Randomized Clinical Trial.
There, for the first time ever, we find a Chantix clinical trial blinding integrity assessment. Data from that assessment raises concern that all Chantix findings to date have been infected and distorted by the collision between assignment expectations and assignment awareness.
Almost 90% of participants assigned to standard Chantix treatment in the Hawk 2011 study had made at least one prior quitting attempt (male=91%, female=88%). What we don't know is the percentage who made five or even ten prior tries.
What makes the Hawk 2011 blinding assessment findings so important is that participants were asked to guess their assignment to Chantix or placebo a week prior to their target quitting date. Thus, it's difficult to contend that Chantix's worth as a quitting aid had somehow unmasked or biased guessing.
Far from being blind, 75 percent of participants receiving Chantix correctly identified their assignment a week prior to their target quitting date. According to Dr. Hawk, "We asked them to make a forced choice. That was followed with a 'how sure' question, but our analyses focused on the forced choice."
That awareness driven choice arose from the combination of a laundry list of known Chantix side effects (including nausea, insomnia and abnormal dreams), awareness by females of smoking fewer cigarettes per day (an average of 5 fewer in the extended pre-quitting Chantix use group), and what the study's authors describe as "significant decreases in the rush provided by the first cigarette of the day across the pre-quit period".
Blinding integrity was questioned after the first extended pre-quit Chantix use study (Hajek 2011) had failed to conduct an assessment. There, it was correctly hypothesized that in Chantix trials that "failure of the blind was likely greater in the active than placebo group."
Surprisingly, even within Hawk 2011's pre-quitting placebo group, 61% correctly identified their assignment. But how? Apart from the symptoms learned during informed consent, Pfizer's Chantix television ads review a host of symptoms. How many times were participants bombarded by such ads stating that, "The most common side effect is nausea. Patients also reported trouble sleeping and vivid, unusual or strange dreams"?
A burning remaining question following Hawk 2011 is, how much would the placebo group's 61 percent pre-quitting assignment awareness have climbed within 72 hours of being told to quit smoking, if not then reassigned to cross-over and begin taking Chantix (as occurred in Hawk)?
Within 24 hours of quitting, what percentage would have recognized onset of the same level of anxiety, anger, dysphoria, concentration difficulty and sleep fragmentation seen during previous failed attempts? How many would have grown frustrated at recognizing their placebo assignment, so frustrated that they would have throw in the towel and relapsed?
Additionally, upon being commanded to quit, how much higher would the extended Chantix group's 75 percent Chantix assignment belief have climbed upon discovery that their normal and expected withdrawal syndrome had significantly changed or was absent? Six years after Chantix's arrival we still don't know.
Why until now did Pfizer ignore assessment of blinding integrity? Did Pfizer know in advance that its varenicline studies would not be blind and that blinding bias would impact performance? And its important and when adjusted for can alter a study's outcome.
Quoting from the Dar 2005 study, "The present secondary analysis of the data elucidates these placebo effects by showing that reduction of smoking was strongly related to participants beliefs about their drug assignment. Smoking reduction was larger in those who believed that they had received nicotine compared with those who believed they had received placebo, regardless of actual drug assignment. Moreover, after adjustment to perceived drug assignment, the association between actual drug assignment and smoking reduction was no longer statistically significant."
Approximately 80% of the placebo group members in varenicline studies relapse to smoking within two weeks. A brief blinding assessment within two weeks could have quickly and easily revealed each participant's assignment belief.
It makes you wonder if anyone at the FDA gives a hoot about blinding integrity in quitting product trials. Has assignment awareness destroyed the credibility and utility of all Chantix study findings to date? Does any government health official care?
The next time you hear a pharm industry commercial suggest that quitting cold turkey is nearly impossible, take your own survey of all long-term ex-smokers who have been off of all sources of nicotine and all cessation dopamine enhancing chemicals for at least one year. Once you complete your own survey we invite you to explore WhyQuit and the wonderful world of educated and supported abrupt nicotine cessation. Quitting need not be a life threatening event. There is still only one rule that if followed offers a 100% guarantee of success to all ... no nicotine, just one day at a time ... Never Take Another Puff, Dip or Chew!
I, John R. Polito, am solely responsible for the content of this article. Any errors brought to my attention will be immediately corrected.