
FDA Chantix handling betrayed public health
John R. Polito
Analysis of a February 2008 study suggests 10 of 11 Chantix users may relapse within six months
 Marketed in the U.S. as Chantix and elsewhere as Champix, February 2008 was an economic disaster for Pfizer's new quit smoking pill varenicline and a credibility nightmare for the U.S. Food and Drug Administration (FDA).
Marketed in the U.S. as Chantix and elsewhere as Champix, February 2008 was an economic disaster for Pfizer's new quit smoking pill varenicline and a credibility nightmare for the U.S. Food and Drug Administration (FDA).
On February 1, the FDA was compelled to issue warnings "regarding serious neuropsychiatric symptoms experienced in patients taking Chantix." On February 8 the FDA awoke to serious warning signs that Chantix's potential worth as a stand-alone quitting product is likely dramatically lower than the FDA has led smokers to believe.
A sleeping watchdog, arguably there is no organization in America that has done more to destroy effective smoking cessation than the FDA. Whether the result of incompetence, misplaced loyalty, apathy, corruption or some combination, millions of smokers are needlessly dying.
Their dying is not because of a lack of desire as 42.5% endure a quitting attempt of at least one day each year. It's because of reliance and misplaced trust upon an agency that seems hellbent on approving quitting aids that are less effective than quitting without them, and determined to destroy confidence in their quitting instincts. Replacement nicotine teaches the wrong lesson about nicotine use and interfers with a natural learning process trying to teach the right lesson, the Law of Addiction.
Patch Exposes Emperor's Outfit
A February study in Thorax (Aubin 2008) reports on the first head-to-head competition between Chantix and the nicotine patch. Imagine being a fly on the wall when FDA officials fully grasped the significance of a key, yet intentionally buried study finding. When comparing "7-day point prevalence of abstinence" (whether or not study participants had smoked any cigarettes in the previous week) researchers found that there "were no significant differences" in quit smoking rates between Chantix and nicotine patch users at either 6 months or 1 year.
It's worth saying again, the nicotine patch has proven as effective as Chantix at helping smokers stop smoking at both 6 months and 1 year. If no more effective than NRT products already on the market, and no news of NRT having killed anyone, then why on July 1, 2009 did the FDA warn smokers that Chantix could kill them and that the FDA was no requiring Chantix to carry its highest warning, a black box? Why not just pull it from the market?
Could it be that the FDA doesn't think that Aubin 2008 study is worthy of belief, that it was funded, designed or influenced by one of Pfizer's nicotine patch competitors? No. The study lists eight co-authors: four Pfizer employees, three paid consultants to Pfizer, plus Pfizer funded the study.
But in regard to being worthy of belief, what's incredible is that following the study's ten-week nicotine patch treatment period and twelve-week varenicline treatment period, that "use of NRT during the 9 months of follow-up did not disqualify a subject" from being classified as a successful quitter. Sleep on that.
Heroin addicts are not addicted to needles nor are smokers addicted to cigarettes. They are both addicted to the chemical inside. With nearly 40% of nicotine gum users hooked on the cure, including President Barack Obama, this pharmacology study, as nearly all, screams loud and clear that neither the FDA nor the pharmaceutical industry cares whether or not any prescription or OTC customer ever breaks nicotine's powerful grip upon their brain. It's a fact that delights the rest of the nicotine addiction industry, as it keeps Senator Obama in the family where smoke's speed and bang reign supreme.
What the Aubin study did do was have participants blow into a carbon monoxide detector in order to attempt to verify their assertions that they had not smoked nicotine. The test is fairly accurate in determining whether or not a person has smoked within the previous 4-5 hours, carbon monoxide's chemical half-life. Results indicated that 20.3% of nicotine patch users and 26.1% of varenicline had probably not smoked for at least 4 hours.
But Chantix's modest expired carbon monoxide victory leaves FDA officials having to explain the nicotine patch's rather strong performance of 20% at one-year, a rate three times higher than the patch's 7% six month over-the-counter (OTC) rate, and speculating as to Chantix's rate when used as a stand-alone quitting aid, without record levels of counseling and support, especially in light of mounting alarm over a number of varenicline's 165 potential side effects.
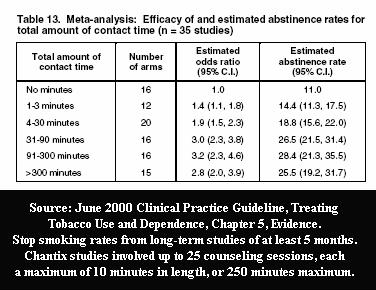 As with Pfizer's earlier varenicline studies, the Aubin 2008 study provided participants with a record setting number of provider counseling/support sessions -- twenty-five -- each involving up to 10 minutes of counseling during every "telephone and clinic visit."
As with Pfizer's earlier varenicline studies, the Aubin 2008 study provided participants with a record setting number of provider counseling/support sessions -- twenty-five -- each involving up to 10 minutes of counseling during every "telephone and clinic visit."
The FDA and Pfizer were intimately familiar with U.S. Clinical Practice Guideline evidence Tables 13 and 14 when approving Pfizer's initial Chantix study designs. Prior to any Chantix study ever, they knew that counseling and support contacts alone, without Chantix, were capable of generating exciting and newsworthy long-term cessation rates.
Guideline Table 13 taught them that up to 25 sessions lasting up to 10 minutes each, providing a maximum total contact time of 250 minutes, could generate long-term quit smoking rates in the neighborhood of 28.4%, affording Chantix (or Billy Bob's Lima Bean Butter) its best chance to appear as successful as possible.
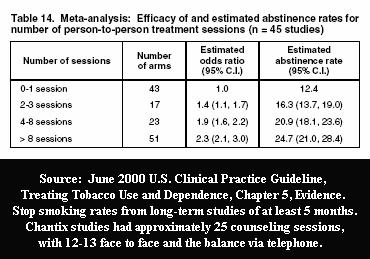 Table 14 taught them that using 12-13 person-to-person counseling sessions, in each Chantix study, should generate long-term quit smoking rates in the neighborhood of 24.7%. They likely suspected that throwing in a dozen extra telephone counseling sessions might generate results worthy of headlining the evening news health segment.
Table 14 taught them that using 12-13 person-to-person counseling sessions, in each Chantix study, should generate long-term quit smoking rates in the neighborhood of 24.7%. They likely suspected that throwing in a dozen extra telephone counseling sessions might generate results worthy of headlining the evening news health segment.
But counseling and support contact time and sessions provided equal benefit to both the Chantix and placebo groups in Pfizer's five initial varenicline studies, correct? Incorrect. Study history had taught the FDA to expect as many as 80% of placebo group members to relapse within two weeks, making it impossible for them to benefit from weekly ongoing support.
What we don't know is what would have happened if the one or two counseling sessions attended by placebo group quitters prior to the vast majority relapsing, had focused on teaching them how to navigate full-blown withdrawal instead of the importance of taking a dopamine enhancement pill, a pill designed to postpone sensing diminished dopamine flow.
Like the war in Iraq, there appears to have been little planning beyond the excitement of varenicline's initial victories. What the FDA did not then know was how Chantix would fare in head-to-head competition against the most studied over-the-counter (OTC) quitting aid ever, the nicotine patch.
A decade earlier, on July 3 and August 2, 1996, when issuing press releases sharing news of approval of OTC status for nicotine patches, the FDA appears to have been guilty of bending truth in order to aid sales. Who benefits, who is misled, but more importantly, what logic is there in giving quitting success rates while still in the process of using a product to quit? The FDA declared the Nicoderm CQ patch's one-month quitting rate to be 19% when the treatment period for wearing the patch was 8 to 10 weeks, and the Nicotrol patch's one month rate to be 20% when the period for wearing it was six weeks? Alarms should have been sounding as the FDA approved OTC patch status based upon rates that were not superior to historic "on your own" quitting rates.
The FDA cannot deny its participation in bashing the quitter's natural instincts to quit cold turkey or, as with Chantix, leading smokers to falsely believe they could expect to experience the same quitting rates as generated in pharmacology studies in which quitting products, including the patch, were often heavily clothed in support protocols. It also cannot deny knowing how horribly the patch performed in over-the-counter studies at six months (not one year) when forced to stand on its own.
Imagine the faces of FDA executives while reading the Aubin 2008 study, when it hit them that in real-world use the nicotine patch, when used as a stand-alone quitting aid, unaccompanied by rich counseling and support, generated an extremely dismal six-month rate of just 7%, and vastly worse for second-time patch users.
Imagine the throat lumps upon realizing that if Chantix's "real-world" performance follows suit, proportionally, that its six-month rate would be in the neighborhood of 9% (20/26=7/9), at least a full percentage points worse than an unaided quitter's own natural odds of 10-11%, a rate evidenced by historic control group rates presented in the US Guideline.
Should Chantix Be on the Market?
The FDA is supposed to stand as gatekeeper between those whose products are health scams that would rob Americans of their money or actually do them harm, and those which are safe and would benefit public health. With a batting average of less than zero, it's hard to imagine the FDA's record in evaluating quit smoking products possibly getting worse. But with Chantix, millions of Americans have front row seats.
On October 27, 2006 the CDC was forced to report that the U.S. smoking rate stalled at 21% during 2005, the first year since 1997 that the rate failed to decline. This occurs at a time when a wide array of FDA approved nicotine replacement therapy (NRT) products scream the laughable and proven falsehood that they are twice as "effective" as quitting cold turkey.

The FDA knew that a June 2000 survey review published in the Journal of the American Medical Association had concluded that "NRT appears no longer effective in increasing long-term successful cessation in California smokers." It knew that in nearly every "real-world" quitting method survey conducted to date, that those quitting without FDA approved pharmacology products performed better than those quitting with them, including long-term results from a 2006 survey by the National Cancer Institute and a 2006 survey of patients of Australian family practice physicians.
The FDA's February 1, 2008 "safety alert" states that symptoms seen in Chantix users include "changes in behavior, agitation, depressed mood, suicidal ideation, and attempted and completed suicide." Should the FDA permit Pfizer to continue to sell Chantix if reports ofscores of suicides are attributable to it and Chantix's long-term benefit as a stand-alone quitting aid is likely slightly less than quitting without it?
The FDA's July 1, 2009 Chantix and Zyban consumer alert states that the FDA "is requiring manufacturers to put a Boxed Warning on the prescribing information for the smoking cessation drugs Chantix (varenicline) and Zyban (buproprion). The warning will highlight the risk of serious mental health events including changes in behavior, depressed mood, hostility, and suicidal thoughts when taking these drugs."
Looking at the FDA's track record in failing to correct erroneous cessation product decisions, the safe bet is that even under President Obama's administration that the FDA will continue to align with pharmaceutical industry economic interests.
Is FDA Shamming Smokers or Just Blind?
On the issue of "clinical efficacy" (in cessation studies, normally how well a quitting product performs against quitters "unknowingly" assigned to use an inert placebo product or device), since Mooney's 2004 "Blind Spot" study, the FDA has been unable to deny full awareness that attempts to blind participants as to active or placebo group assignment in randomized NRT studies have been a sham.
Blinding or masking is used in pharmacology studies to attempt to remove the influence of participant knowledge, perceptions and beliefs, so that expectations do not affect results. In clinical trials, a study is blind if participants cannot tell whether or not they have been randomly assigned to receive an inert placebo or the product being evaluated. In smoking cessation studies, expectations abound, with many created by extremely sloppy researchers, who appear more concerned with catering to the interests of those funding their research than making meaningful contributions to science.
For example, instead of avoiding fostering expectations by simply recruiting for a "quit smoking study" that could involve a wide array of quit smoking methods (group support, counseling, hypnotherapy, acupuncture or evaluation of a new product or medication), many studies entice and tease smokers into joining by creating super-sized expectations. Those evaluating pharmaceutical products often advertise that the study will "test a new medication designed to help people quit smoking," tip them off to the name of the medicine or before getting them to the study site, alert them to the concern that they may be given a placebo instead of medicine.
If your instincts told you that quitting cold turkey was the way to go, but you were hoping to locate a quality counseling or support program to dramatically increase your odds, what would your reaction be to a study flyer recruiting smokers for a "medicine" study? Your likely answer is the reason why clinical "medication" quitting trials have almost no relevance to the expectations or performance of cold turkey quitters. Yet the FDA knowingly allows Pfizer advertising to falsely suggest victory over cold turkey quitters, quitters it knows were not present and did not participate.
Imagine being a smoker who had repeatedly tried quitting and failed but this time deciding to join a "medicine" study, and hoping to receive "real" medicine, not a placebo. Would you stick around and allow yourself to continue to be toyed with if arrival of full-blown withdrawal totally shattered your expectations? On the flip side, would you stay and unknowingly subject yourself to the benefit of 25 upcoming counseling sessions if your expectations were fulfilled and your withdrawal experience seemed less intense than with any prior quitting attempt?
The science base and core validity of more than 100 quitting product studies rests upon two simple questions: (1) were those studies truly blind and (2) if not, did blinding failures influence findings?
It is an undeniable truth that any drug addict with a significant quitting history knows what it feels like to be thrown into full-blown withdrawal. Is it fraud to pretend they can't? Shouldn't they also be expected to recognize a less intense withdrawal experience?
As I wrote in Canada's leading medical journal in November 2008, "the fact that cessation with pharmacological treatment has failed to be more successful than cessation without such treatment in nearly all of real-world surveys conducted to date, strongly suggests that the pharmacologic treatment of chemical dependency may be the only known research area in which blinding is impossible."
The FDA must by now be fully aware that drug addiction may be the only pharmacology study area where blinding actually punishes participants, in making them vastly worse than when they arrived, by throwing them into full-blown drug withdrawal. It also knows that efficacy findings in drug addiction studies evidence fulfilled and frustrated expectations, not clinical efficacy and not "real-world" effectiveness.
The very first nicotine gum study ever, the 1971 Ohlin study, was a play-book in how to make money. It alerted the pharmaceutical industry that counseling and support were probably superior to nicotine gum alone, to never allow NRT to go it alone in challenging counseling, that nicotine gum shows promise in competing with placebo gum users, but that blinding is a significant concern.
In the years following Ohlin, the FDA witnessed study blinding concerns grow so out of hand that NRT researchers resorted to the extreme of putting small amounts of nicotine into placebo devices. The practice dates back to early nicotine gum studies, as far back as the 1982 Jarvis study. There, 2mg nicotine chewing-gum "was compared with a placebo containing 1 mg nicotine, unbuffered" (also see Areechon 1988 and Clavel-Chapelon 1997).
This dark chapter in cessation study history is understandably rarely talked about. Some researchers cannot tell you whether or not the industry supplied placebo device used in their study contained nicotine. We do not know how widespread the practice was. The only research we have shows that smokers are able to discriminate the presence of nicotine at levels far below the amount delivered by cigarettes (also see Leischow 1995).
Normally a cold turkey quitter's brain is 100% nicotine free within three days of ending all nicotine use, at which point withdrawal peaks in intensity and they gradually begin feeling better. Imagine sentencing them to live in a state of chronic perpetual withdrawal for up to 12 weeks by feeding them, via placebo, the nicotine equivalent of smoking up to three cigarettes a day, and even more if they daily chewed the recommended number of pieces of gum.
The text of some nicotine patch studies reveals that up to 3 mg of nicotine was used in placebo patches. The Campbell 1996 patch study states that the placebo group was "supplied placebo patches containing 13% of the nicotine found in the active group," which would appear to be 3 mg for the 21mg patch group. The Sonderskov 1997 nicotine patch study, in which only 4.2% of placebo group members in the 21mg patch arm were still not smoking at six months, recites that "placebo patches contained a pharmacologically negligible amount of nicotine," without revealing how much (also see Ahluwalia 1998, Abelin 1989 and Richmond 1994).
The FDA has had four years to react to Mooney's "Blind Spot" study-pronouncement that NRT studies were generally not blind as claimed. Mooney concludes by warning the FDA that "clinical trials of NRT should uniformly test the integrity of study blinds. Moreover, if blindness failure is observed, subsequent efforts should be made to determine if blindness failure is related to study outcome and, if so, to provide an estimate of treatment outcome adjusted for blindness bias. Without these methods and analyses, the validity of NRT clinical trial results could be questioned."
Mooney was followed by a 2005 study by Dar which found that 3.3 times as many placebo group members correctly guessed that they had received placebo (54.5%) as guessed that they had received nicotine (16.4%). Although the Dar study focused on smoking reduction, Tonnesen's 1993 nicotine inhaler quitting study produced strikingly similar placebo group findings in that 3.8 times as many in the placebo group correctly guessed placebo (58%) as guessed nicotine (15%). Among inhaler users, Tonnesen found that 3.5 times more correctly guessed inhaler (46%) as guessed placebo (13%).
It is as if the FDA, instead of immediately banning placebo use in drug addiction studies, has instead chosen to stick its head in the sand. It's as if it feels that mastery of how blinding has been a licence to steal is simply too challenging for journalists to wrap their brains, thoughts and words around.
Simply changing dopamine pathway stimulants from nicotine to varenicline does not relieve the FDA of study integrity obligations, as varenicline generates up to 60% of the dopamine flow that nicotine would if setting on the exact same receptors. In fact, in Chantix studies, the placebo group's attempt to stop smoking on day #8 (as instructed by Pfizer) puts them exactly where they were in NRT studies on day #1, punished by the onset of early withdrawal.
Actually, it's likely that blinding concerns among those assigned to receive varenicline are more profound than in NRT trials. Prior to quitting smoking on day #8, many had actual notice that a foreign chemical was at work inside their brain. Thousands upon thousands of times they had each sensed a powerful dopamine "aaah" explosion inside their brain's dopamine pathways within 8-10 of that first puff of nicotine. But now varenicline was sitting on those same receptors, blocking nicotine from generating its customary "aaah."
For them, if smoking nicotine was now akin to smoking a carrot, and they sensed and knew this prior to ever quitting smoking on day eight, has the FDA been honest with American smokers in boasting about meaningless victories over placebo users, in studies it clearly knew were not blind?
Scientific integrity demands that the FDA refuse to accept any new cessation pharmacology study finding unless accompanied by an independent blinding evaluation, as outlined by Mooney. Study blindness should be evaluated at a point near where most placebo group members grow frustrated and relapse, usually by the two-week mark, while memories are fresh, beliefs untainted, and all participants still easy to contact.
Also, why rely on the fox for accurate counts of hen house hens? Like FDA meat inspections, I submit that the FDA should itself be responsible for evaluating the core integrity of any study being relied upon for agency approval of any new medication.
Not Just Enabler But Partner
The totality of the FDA's handling of varenicline should alarm Americans. Who does it represent, pharmaceutical interests or pubic health? The FDA admits to having given Chantix a "priority FDA review," but in doing so made human Guinea pigs of nearly all of the 28% of study applicants Pfizer intentionally excluded from its clinical trials.
They included nearly all having any clinically significant medical disease, including psychiatric ailments such as major depression, panic disorder, psychosis, bipolar disorder or alcohol or other drug abuse.
The FDA could easily have conditioned approval on all Pfizer advertising alerting every group excluded from study participation of the fact that there was as yet no evidence that Chantix use was safety or effectiveness for them, but it didn't.
Online FDA documents show that the FDA had substantial involvement in pre-approving Pfizer's varenicline studies. The FDA knew in advance that Pfizer was not designing these studies to show varenicline's true worth, if any, as a stand-alone quitting product, but to produce the highest quitting rate possible by submerging varenicline in the most persistent counseling and support regime ever.
The FDA knew when it approved Pfizer's study plans, plans that generated an average one year quit smoking rate of 21%, that it was unlikely that any American Chantix users would receive 24-25 counseling or support sessions from their prescribing physician.
But for some reason that didn't seem to matter to FDA approving officials. Instead, the FDA continues to allow the pharmaceutical industry to use these exact same bait and switch study tactics on American smokers, tactics that have made the industry billions these past 24 years.
What if Pfizer actually had to pay to deliver the performance its advertising impliedly promises, at least a dozen live local counseling sessions and a dozen support telephone calls? Imagine the price. Which Pfizer ad alerts smokers to the fact that most of Chantix's success is probably not due to Chantix but counseling and support?
When nicotine gum was first evaluated in the early 80s, it was so heavily wrapped in counseling, support and/or intense study protocols that results electrified the cessation world. The 1976 Russell study found 23% of nicotine gum users still not smoking at one year, the 1980 Raw study produced a whopping 38% rate, in 1982 Jarvis saw 31%, 1983 Schneider 30%, 1984 Hialmarson 29%, 1986 Daughton 31%, 1987 Kornitzer 32%, and the 1989 Tonnesen study boasted an unbelievable 44% one-year quit smoking rate.
Yet the FDA's May 11, 2006 Chantix approval press release, the release it knew would provide the factual foundation for hundreds of Chantix news stories establishing initial smoker Chantix beliefs, failed to once mention that Pfizer's studies had excluded nearly all having clinically significant medical conditions or that they involved the greatest number of counseling and support sessions ever seen in studies designed to gain FDA cessation product approval.
The FDA did not alert smokers that Pfizer's initial Chantix studies were such a mess that participants who started using NRT after ending 12 weeks of varenicline use were knowingly counted among successful Chantix quitters. There was no mention that there was no examination of the blood, urine or saliva of Chantix quitters to see if any had actually broken free of nicotine, that Chantix studies were not about arresting chemical dependency upon nicotine but about ending use of just one nicotine delivery vehicle, smoke.
Instead, the FDA knowingly hid critical information, important to informed decision making. It fed smokers false hope and unrealistic expectations by proclaiming that Chantix had "significant potential benefit to public health," that "Chantix therapy has proven to be effective in smokers motivated to quit," that "effectiveness of Chantix in smoking cessation was demonstrated in six clinical trials," that it "was shown to be superior to placebo," and that users "were also more successful in giving up smoking than patients treated with Zyban."
System Overhaul Needed
What's needed is a major overhaul of America's drug approval process, including research ethics. We need to devise a new means of either funding all studies being relied upon in approving medications, or guaranteeing independent oversight of the study process. We cannot continue to expect a for-profit industry to be truthful with government about what works and what does not. We should not allow net profit analysis to control research priorities, including which quitting method is evaluated?
Why allow stacking of advisory panels by industry consultants, researchers and grant recipients with no history of ever once having stood-up to the industry, of having demonstrated independence by having at least nibbled on the hand that feeds them? We watched at their influence transformed what could and should have been a wonderful guide to teaching physicians how to counsel and support patient nicotine cessation into a worthless quitting product sales catalog.
But industry influence has been even bolder. In June 2000 it rewrote official government cessation policy so as to declare all attempts to quit without pharmacology as in violation of it. Today's policy reads, "Numerous effective pharmacotherapies for smoking cessation now exist. Except in the presence of contraindications, these should be used with all patients attempting to quit smoking."
Imagine declaring educated and supported cold turkey quitting, illegal. Although the Wall Street Journal has questioned the ethics of the fox residing in the hen house, corporations are driven by profits not conscience.
In regard to the bigger picture, what incentive does the pharmaceutical industry have to develop medications that treat conditions that only afflict tens of thousands of Americans? What incentive is there to study the health benefits of exercise, diet, sunshine, aspirin or even education-oriented nicotine cessation programs, or fairly and honestly contrast their efficacy or effectiveness to approved medications when there are zero profits to be made, and product credibility could be lost?
"Absence of evidence is not evidence of absence." The system is broken when our nation's medicine approval agency is so aligned with pharmaceutical interests that it aids and abets in destroying competition. Reflect on the insanity of declaring the quitting method used by nearly 90% of successful long-term ex-smokers to be in violation of government policy. Imagine two decades of marketing relentlessly pounding home the message that their natural instincts are wrong.
Government holds a massive incentive hammer that its leaders are afraid to use. What clause in the U.S. Constitution forbids government from researching, producing, and either licensing or actually distributing medicine? Together we build roads but not medicines to keep travelers healthy and alive. Why?
And system overhaul can't happen too soon. Not only do those on the inside face serious integrity issues, it's getting hard to identify any clinical pharmacology researcher on the outside whose mind isn't already pickled and living the principle, "first, do no harm to pharmaceutical financial interests."
Most pretend independence but know full-well that shedding denial and being totally truthful would instantly destroy their pharmaceutical industry financial relationships, depriving them of a dependable source of future income.
Imagine devoting a major portion of your life to inventing excuses as to why cessation pharmacology falls flat on its face once outside the trial clinic's doors. Imagine being deprived of the right to tell the truth, or having to write studies so as to omit, hide, minimize or rationalize findings contrary to industry economic interests, or being asked to shelve the study altogether.
OMB Watch reports that Congress is about to take a closer look at America's drug approval process. Hopefully, both cessation pharmacology researchers and FDA workers will find the courage to step forward, the way pharmaceutical sales reps are doing (see Dr. Drug Rep., Olsen, Zyprexa) and provide Congress the insights needed to destroy a "culture of approval" that elevates corporate well-being above safety and effectiveness.
FDA Tobacco Regulation Would Further Damage FDA
It's ironic that the FDA's Chairman opposes pending legislation - "The Family Smoking Prevention and Tobacco Control Act" - that would grant the FDA jurisdiction to regulate tobacco, because this time he's dead right. Although the bills are championed by Kennedy in the Senate (S.625) and Waxman in the House (H.R.1108) they are loaded with tobacco industry gifts, as it is widely believed that Philip Morris USA had a big hand in actually write them.
But Kennedy and Waxman have every right to be extremely confused right now. In 1984 they watched the FDA jump at the chance to regulate pharmaceutical grade nicotine and every new industry nicotine delivery device since. They watched the FDA, with a straight face, re-label one of earth's most potent toxins "medicine," a natural insecticide that drop for drop is more lethal (LD50 of 60mg) than strychnine (75mg), diamondback rattlesnake venom (100mg) or cyanide (500mg), while calling its use "therapy."
Although the FDA invited this mess by lacking the integrity to put an end to bait and switch study practices and ignoring a lack of blinding integrity, Kennedy and Waxman need to be careful what they wish for.
In 2003, GSK consultants reported that 37% of nicotine gum users were hooked on the cure and there is credible talk of a host of new, cleaner nicotine delivery devices in the pipeline. The days may be numbered for the dirtiest drug delivery device the world has ever known. Why give cigarettes an official government birth certificate that, under this bill, guarantees their survival until such century that Congress votes to ban them?
Philip Morris' own website openly admits that "there is no safe cigarette." If after spending billions and decades on research, if Philip Morris has surrendered to the fact that it cannot produce a safe cigarette, then why order the FDA to spend billions of precious taxpayer dollars plowing the exact same ground (dollars that could have been spent inspecting meat and ensuring study integrity), only to reach the same conclusion?
Why compel 9,000 FDA employees, watchdogs we want getting excited about any product related death, to accept regulatory responsibility for more than 400,000 annual smoking related deaths, deaths caused by a product that all agree cannot be made safe? Why compel the FDA to live under legislation that forbids it from ending the slaughter?
Reflect upon the precious waste, sanity, destroyed ideals and shattered faith of Congress ordering the guardian of our nation's foods and medicines to devote substantial resources to trying to make chemical enslavement to smoking a super toxin that eats brain gray matter more acceptable by society. Tobacco industry thinking is light years ahead of Congress. Their laughter grows with each new bill sponsor that's added.
Imagine the message sent to children and teens if Philip Morris and R.J. Reynolds were to now and then remind them that the cigarette's safety is regulated by the exact same agency that protects the foods they eat. Imagine the injustice done when jury room discussions in tobacco products liability actions turn to the fact that the FDA is responsible for regulating cigarette safety. Behind Kennedy and Waxman's well-intentioned backs, industry laughter must be hard to contain.
Philip Morris does not want product liability law to work its course. Instead it wants the protections afforded by these bills, which arguably strip states of their current power to outlaw cigarette sales [see Sec.907(b)(3)(A)]. Philip Morris wants to destroy such power, not watch states gradually awaken to it.
Both bills forbid the FDA from requiring removal of all nicotine from cigarettes [see Sec.907(b)(3)(B)], from banning the sale of cigarettes [see Sec.907(b)(3)(A)], from requiring that cigarettes only be sold in stand-alone stores to which youth are denied access [see Sec.906(d)(3)(A)(i)], and from increasing the national smoking age to a minimum of 19 in order to deny an army of 18-year-old high school students the ability to purchase cigarettes for underclassmen [see Sec.906(d)(3)(A)(ii)].
A more accurate name for the bills would have been the "The Family Smoking and Youth Nicotine Addiction Act." Although the intentions of most backing these bills are honorable, their lack of understanding of basic industry youth enslavement tactics could result in needless decades of Congressionally sanctioned war on America's youth.
Some legislation supporters have resorted to falsehoods, including suggesting that "Big Tobacco" is trying to kill the bills when the cigarette company holding a 51% share of the U.S. cigarette market is working hard to realize the bill's protections.
But we have absolutely no right to expect Senator Kennedy, Representative Waxman and other members of Congress to see deeply buried truths after 24 years of the FDA having pounded home the false message that nicotine is medicine, its use therapy and it saves lives.
It's my hope that they will devote time to learning more about the destructive potential of this most amazing chemical, work to repair the FDA's integrity, void current U.S. cessation policy, and amend 15 USC §1334 to repeal preemption, allowing states to immediately require point-of-sale signs teaching youth the truth about why smokers smoke, because they must, because a rising tide of insula driven craves and anxieties begin to hurt when they don't.
Many credit the Canadian cigarette pack addiction warning label as having contributed to a dramatic decline in Canadian youth smoking rates. Since 2000 it has read, "Warning - cigarettes are highly addictive - studies have shown that tobacco can be harder to quit than heroin or cocaine." It was never Congress' intent in enacting 15 USC §1334 to forbid cities and states from passing ordinances and laws requiring that all tobacco sales locations warn youth about nicotine's true power. But you can bet the farm that it was a tobacco industry objective.
There has never been a U.S. addiction warning label. Looking at the weak, watered-down, half-truth warning proposed by these bills in Sec.201 which states, "Warning: Cigarettes are addictive," I cannot accept that it is Congress' intent that American youth not be told how addictive (highly addictive) or provided any basis for comparison with other drugs of addiction. Looking at the addiction warning on Philip Morris' website, I do accept that the proposed youth warning is fully acceptable to it.
The lines between pharmaceutical industry nicotine and tobacco industry nicotine and quickly blurring, as are their marketing practices. It is no secret that they work in partnership. Try to locate even one NRT, Zyban or Chantix ad telling smokers that smoking is bad for them. There are none and it isn't likely there will be. Imagine a billion-dollar industry that cannot motivate purchases by telling customers why it is important to quit.
The system is clearly broken and a meltdown of FDA integrity was central in breaking it. But do we care? If so, the time for action is now.

