
13th World Conference on Tobacco or Health drenched in nicotine
Keenly aware of smoking's massive annual slaughter and in search of help, government health officials from around the globe will descend upon Washington DC from July 12-15 for the 13th World Conference on Tobacco or Health. What they'll find instead is that the conference's two corporate sponsors -- GlaxoSmithKline and Pfizer -- have produced a well orchestrated commercial designed to convince them that government subsidized nicotine is the answer - replacement nicotine or NRT. What they won't hear is the truth, that replacement nicotine has never proven effective in any real-world setting and likely never will.
The California tobacco survey, the Minnesota insurance survey, Quebec Quit and Win, the Tobacco in London survey, Western Maryland, UK NHS Smoking Cessation Services, Australia family practice survey, two decades after its 1984 introduction NRT does not have a single real-world performance victory, none. But that isn't stopping the pharmaceutical industry from encouraging health officials to waste precious resources purchasing a worthless remedy.
NRT Clinical Studies Were Not Blind and Are Not Trustworthy
The reason NRT will never be effective in head-to-head real-world competition is that the expectations of cold turkey quitters to abruptly end all nicotine use are beyond the ability of the pharmaceutical industry to exclude, redefine, tease, torment, play upon, frustrate, defeat or destroy.
The clinical lesson kept quiet by the pharmaceutical industry and its army of loyal research consultants is that clinical efficacy studies were an expectations nightmare. Study participants joined in hopes of receiving weeks or months of free replacement nicotine. Instead of NRT clinical odds ratio victories evidencing NRT efficacy they reflect the frustration and fulfillment of the nicotine addict's nicotine expectations.
Nicotine is a psychoactive chemical and a substantial percentage of participants knew what it felt like when their dopamine/adrenaline high was or was not replaced. This isn't news to a replacement nicotine industry that appears to be operating from the tobacco industry's nicotine play-book. Researchers found themselves resorting to the extreme of toying with small amounts of nicotine as a placebo device masking agent as early as a 1982 nicotine gum study. The practice is also noted in a number of nicotine patch studies (see Campbell 1996, Sonderskov 1997 and Ahluwalia 2002).
Clinical efficacy and community effectiveness are two entirely different standards. According to an August 2004 article by Dr. Lois Biener, PhD, Senior Research Fellow, University of Massachusetts, "the effectiveness of NRT in the general population has not been established. In spite of the fact that NRT and other drugs are included in the Public Health Service guidelines, their efficacy has only been demonstrated in carefully controlled clinical trials. Evidence of their effectiveness in general population has been difficult to find."
Dr. Biener is one of two Conference presenters who have demonstrated the courage to speak truth to pharmaceutical industry muscle, money and influence. It isn't as common as integrity would hope. The other is Dr. John Pierce, PhD, Professor of Family and Preventive Medicine, University of California, San Diego.
Dr. Pierce analyzed seven years of data from the California Smoker's Survey, one of the world's largest. His study, published in the September 11, 2002 issue of the Journal of the American Medical Association, concluded that "NRT appears no longer effective in increasing long-term successful cessation in California smokers."
There is growing awareness that highly manipulated clinical studies involving chemicals which alter perception, heart rate, extremity temperature, mood and behavior cannot and must not be accepted at face value. Equally important are real-world performance evaluations, a step that both GlaxoSmithKline and Pfizer know they must avoid and belittle as unscientific if their golden nicotine goose is to continue laying golden eggs.
More than two years have passed since Mooney reviewed the blinding procedures associated with 73 "allegedly" double-blind NRT studies. Published in the June 2004 issue of Addictive Behaviors, he found that clinical NRT studies were not generally blind as claimed in that "subjects accurately judged treatment assignment at a rate significantly above chance."
In clinical trials nicotine expectations were either frustrated or fulfilled shortly after randomization. Most placebo group members dropped out in the first few weeks of clinical studies. The NRT industry has had two full years to conduct rather simple NRT blinding evaluations to prove that clinical NRT study results do not reflect the worst junk science and greatest smoker hoax the world has ever seen. The industry has had nearly four years since Dr. Pierce's ineffectiveness survey finding to produce its own survey showing effectiveness but it hasn't and it won't. Why? Because it can't.
Instead, the 13th World Conference on Tobacco or Health will be used to introduce new excuses for NRT's dismal performance and to make implied promises of future performance that cannot and will not be kept. What health policymakers should be asking is how many priceless periods of cessation confidence has NRT already squandered? How many lives have needlessly been lost?
False Advertising
GlaxoSmithKline's Quit.com site asserts, "In general, NRTs have been shown to double a smoker's chances of quitting versus 'cold turkey.'" Pfizer's Nicotrol website asserts, "Studies have shown that nicotine replacement therapy can double a smoker's chances of quitting versus cold turkey."
GlaxoSmithKline and Pfizer should be compelled to immediately identify any study which invited those dreaming of quitting cold turkey to compete against those wanting and seeking free replacement nicotine. Given that NRT has never once prevailed over "cold turkey" quitters in any head-to-head real-world survey and that those wanting to quit "cold turkey" were never invited to challenge NRT in any clinical trial, aren't both GlaxoSmithKline and Pfizer -- the pharmaceutical industry sponsors of the World Conference -- engaged in open and intentional smoker deception?
Nicotine Being Painted as Helpful, Enjoyable and Safe
Look what curious youth and smokers are reading about smoking nicotine at GlaxoSmithKline's Nicorette website. "Smoking stimulates chemicals in your brain that appear to enhance awareness and concentration. It increases dopamine levels, which improves your mood. It even increases the levels of some hormones, including adrenaline. This is why cold-turkey attempts seldom work. But Nicorette helps you control cravings, while gradually weaning your body from nicotine."
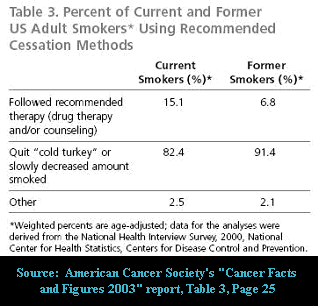 Momentarily overlook the fact that today almost all successful quitters around the globe are quitting cold turkey (80 to 90%). Instead, focus on the allure of the nicotine benefits suggested. What visitors are not told is that GlaxoSmithKline has determined that at least 36.6% of all current nicotine gum users are chronic long-term users of greater than 6 months ( Tobacco Control, Nov. 2003).
Momentarily overlook the fact that today almost all successful quitters around the globe are quitting cold turkey (80 to 90%). Instead, focus on the allure of the nicotine benefits suggested. What visitors are not told is that GlaxoSmithKline has determined that at least 36.6% of all current nicotine gum users are chronic long-term users of greater than 6 months ( Tobacco Control, Nov. 2003).
What is not shared are recent studies evidencing that nicotine is a major player in the harms caused by smoking. It has now been linked to chronic depression, hardening of the arteries, accelerated tumor grow rates, to rendering chemotherapy substantially less effective, memory impairment and early dementia.
United Kingdom NRT industry consultants are expected to boast to the World Conference that their nation recently approved NRT for both child smokers above age 12 and pregnant smokers. According to Professor Theodore Slotkin with the Department of Pharmacology and Cancer Biology at Duke University Medical Center it's a recipe for disaster.
"There is abundant evidence that the major problem for fetal development is exposure to nicotine rather than other components of cigarette smoke." "NRT, especially by transdermal patch, delivers more nicotine to the fetus than smoking does."
A March 2003 study published in Reproductive Toxicology found that the nicotine concentration in the brains of fetal mice were 2.5 times greater than the nicotine concentration found in the mother's bloodstream when nicotine was continuously administrated, as would be the case with the nicotine patch. A pregnant smoker need only imagine what it would be like if her mind were trapped and forced to constantly endure 2.5% more nicotine than normal.
"The patch is the 'easiest' NRT approach, and it turns out that this is the absolute worst form of nicotine administration for the fetus. Essentially, achieving a continuous steady-state plasma level of nicotine in the mother removes the protective effect of the placenta (delay of entry to fetus, partial catabolism of nicotine) because all water spaces become saturated with nicotine," explains Slotkin.
Imagine giving a thirteen year-old child with a 5mg per day dependency a box of 21mg nicotine patches. How many will share them with friends? What's most disturbing is that the UK government cited no study showing NRT efficacy or effectiveness with youth. To the contrary, the only youth study finding we have concludes, "Nicotine patch therapy plus minimal behavioral intervention does not appear to be effective for treatment of adolescent smokers"(Hurt 2001).
Tobacco Industry/Pharmaceutical Industry Agreements
Imagine spending hundreds of millions on advertising yet never once, in any marketing message, mentioning the horrors that await smokers who fail to quit. Is it coincidence that those selling replacement nicotine continue to fail to mention smoking related diseases or is there an oral or written non-compete agreement between the tobacco and pharmaceutical industries?
Is it coincidence that Philip Morris' website has touted replacement nicotine as a "key" to successful quitting? Is it coincidence that the per use cost of over-the-counter replacement nicotine remains at or near the cost of cigarettes when NRT products are not subject to tobacco excise taxes? What are NRT production costs? Whose team is "Big Pharm" really on, theirs or ours? Is its objective to help smokers, shareholders or "Big Tobacco?"
Failure to Disclose NRT's Actual Quitting Rates
Youth and young adults listening to NRT marketing are being led to believe that quitting with NRT is relatively easy and that NRT products are generating high success rates. This marketing message plays directly into the tobacco industry's hand in actually inviting experimentation, knowing that quitting is easy. Worse yet, the message is false.
Professors Saul Shiffman and John Hughes are both admitted GlaxoSmithKline consultants. In March 2003 they combined and averaged all seven over-the-counter (OTC) nicotine patch and gum studies - the manner in which almost all U.S. NRT is sold and used today - and found that 93% of study participants had relapsed to smoking within six months. Those attending should ask the Conference's sponsors why they have kept OTC NRT's dismal 7% six-month quitting rate a secret these past three years.
Imagine GlaxoSmithKline's consultants establishing that only 7% of OTC patch and gum quitters were still not smoking at six months, while a page at its Nicorette website carries a title which reads, "According to one study, 90 percent of 'cold turkey' quitters start smoking again within six months." Is that not admitting defeat?
Failure to Disclose Second-Time NRT Use Rates
Unlike abrupt nicotine cessation, where the odds of success actually increase with each subsequent attempt (as quitters eventually discover the amazing power of one puff of nicotine to shatter and destroy an ongoing quitting attempt) NRT's already dismal odds of success dramatically decline with repeat NRT use.
All Internet websites advocating the use of NRT keep quiet about the only two nicotine patch studies that have ever examined success rates for second-time patch users. Not knowing the results carry potential of being a life or death issue for true believers of NRT marketing hype, as one study found a 100% six-month failure rate (Tonnesen 1993) and in the other 98.4% relapsed (Gourlay 1995).
A February 2004 study by Shiffman in Addiction boldly concludes "Smokers with a history of past failure of pharmacological treatment have lower success rates without pharmacological treatment, but equally good outcomes with active lozenge treatment."
What the 2004 study abstract fails to reveal is that unlike the 1993 and 1995 studies examining second-time nicotine patch use, Shiffman declared repeat NRT use "effective" after only 6 weeks instead of 6 months. Even worse, nicotine lozenge users in Shiffman's study were given up to 20 free lozenges per day for a period of six full months. Imagine giving alcoholics alcohol via IV bags for 6 months while declaring those still wearing the bags successful quitters at 6 weeks.
But that has not stopped GlaxoSmithKline from using what is primarily abrupt nicotine cessation historical quitting data in an attempt to sell replacement nicotine to those who have already repeatedly tried it and failed. For example, GlaxoSmithKline's Quit.com website asserts, "It is quite common for smokers to make anywhere from three to six quit attempts before achieving success."
With each passing year of NRT use, NRT use recycling becomes more critical as in some nations almost 50% of all smokers have already tried quitting with NRT at least once and failed. Instead of doubling national cessation rates as promised, here in the U.S. cessation has almost ground to a halt.
Not only are health policymakers allowing a completely ineffective line of quitting products to remain on center-stage, they have remained silent for more than two decades as the NRT industry has bashed, trashed and attempted to claim a larger share of the market by all but destroying confidence in the planet's most productive quitting method - abrupt nicotine cessation.
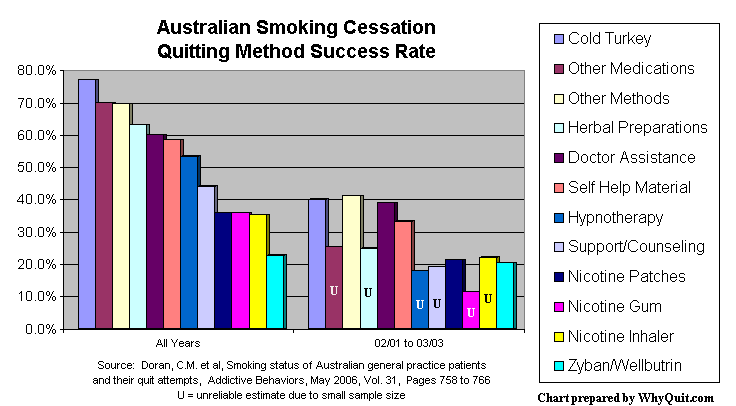
A May 2006 study in Addictive Behaviors analyzed 2002 and 2003 patient quitting method data collected by 1,000 Australian family practice physicians. Our most recent quitting method performance evidence, it found that cold turkey success rates were twice as high as among those relying upon the nicotine patch, gum, inhaler or bupropion (Zyban and Wellbutrin). Not only was cold turkey quitting the most effective method, it was by far the most productive method accounting for 1,942 of 2,207 former smokers, a whopping 88% of all success stories.
Time to Abandon NRT Group Think
If GlaxoSmithKline's 10% at six-month cold turkey figure is correct, even the most ridiculous quitting product imaginable should generate testimonials from 10% of users at six months, so long as it does not somehow undermine the quitter's own natural recovery odds - as does NRT at 7%. It's why no area is more ripe for consumer fraud than smoking cessation.
If current NRT clinical efficacy and real-world effectiveness standards are the benchmark for evaluating a new wave of now arriving pharmaceutical cessation products then the best hope for earth's one billion nicotine dependent humans may well be prayer, and lots of it. We should not trust forward movement while traveling a road built on known and intentionally ignored blinding failures.
Actual drug performance must be elevated above clinical findings, especially when the clinical studies themselves attract a self-seeking population in search of weeks or months of free replacement nicotine, not a population wanting to abruptly end all nicotine use.
Clinical studies have no trouble randomizing quitters with similar expectations. What they cannot do is hide the presence or absence of the dopamine/adrenaline high produced by a powerful psychoactive chemical such as nicotine. What they cannot do is hide the fact that those wanting to abruptly end all nicotine use did not participate in any NRT studies. What they cannot hide is that it is impossible to randomize opposing expectations regarding receipt of a psychoactive substance.
Commentary on the Above Article
- 07/12/06 - Dr. Michael Siegel, M.D., MPH, Professor, Boston University School of Public Health
World Conference Related News Events
- 07/13/06 -GlaxoSmithKline uses World Conference to sell "therapeutic" nicotine
The 4,500 attending the World Conference cannot address NRT ineffectiveness if the Conference's primary sponsor is cramming NRT down their throats. As Dr. Michael Siegel, MD, asserts, "there is a problem of scientific integrity, because you can't objectively discuss the appropriate role of NRT when your chief sponsors, plastered all over everything, are the companies producing and marketing NRT." - 07/12/06 - GlaxoSmithKline claims 5 million quit with NRT without providing any details
GlaxoSmithKline's 5 million NRT quitters assertion has been timed to sell nicotine at the World Conference. Those in attendance should demand facts and hard data supporting this assertion. At a minimum GlaxoSmithKline should willingly provide answers to the following questions:
- Does GSK believe in openness, honesty, and transparency in providing policymakers with all underlying data needed to assess the credibility and significance of GSK's 5 million successful quitter "real-world" performance claim?
- How many of the 5 million quitters were from the U.S.?
- What percentage of U.S. smokers have now attempted quitting with GSK NRT?
- How many U.S. smokers have now attempted quitting with GSK NRT?
- How long did each NRT user need to quit smoking before being counted as a successful NRT quitter?
- How long did each NRT user need to use replacement "therapy" before being counted as a successful quitter?
- How did GSK gather data on successful quitters, how were results verified, and what methods were used to calculate GSK's 5 million successful quitter conclusion?
- Is a copy of that document available?
- If GSK employed some form of survey, what other questions were asked?
- According to GSK's data, what percentage of GSK NRT users are able to quit smoking for at least six months?
- According to GSK's data, what percentage of GSK NRT users are still not smoking at one year?
- According to GSK's data, what percentage of second-time (or subsequent) nicotine patch users are still not smoking at six months?
- According to GSK's data, what percentage of second-time (or subsequent) nicotine gum users are still not smoking at six months?
- According to GSK's data, what percentage of GSK nicotine gum "quitters" are still chronically using nicotine gum at six months?
- According to GSK's data, what percentage of GSK nicotine lozenge "quitters" are still chronically using the nicotine lozenge at six months?
- According to GSK's data what percentage of chronic gum users are still using NRT at one year?
- According to GSK's data what percentage of chronic gum users who were still using nicotine gum at six months but not using it at one year relapsed back to smoking?

