GlaxoSmithKline attacks cold turkey quitting
 Millions of New Year's quit smoking resolutions are about to be tested and those selling quitting cures want a slice of the pie. But how?
Millions of New Year's quit smoking resolutions are about to be tested and those selling quitting cures want a slice of the pie. But how?
GlaxoSmithKline Consumer Healthcare (GSK) markets Nicorette gum, the NicoDerm patch and the Commit nicotine lozenge. On December 5, 2005 GSK issued a press release asserting that almost all cold turkey quitters fail.
What GSK's press release conveniently fails to mention is that almost all of its product users fail too, that rates for repeat nicotine product users are even worse, that NRT studies were not blind as claimed, or that cold turkey was the quitting method used by almost all successful long-term ex-smokers - roughly 90%.
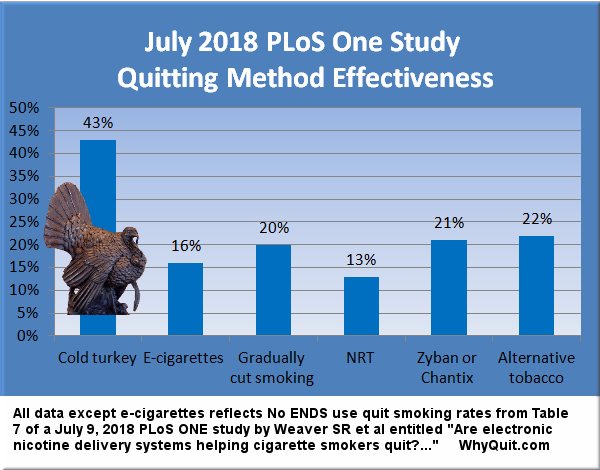
According to GSK's press release, "An estimated three out of four smokers would like to quit and each year about 15 million smokers do quit for at least one day. Yet fewer than 5% of them successfully remain tobacco-free for three to 12 months. Most smokers try to quit using cold turkey even though quitting without the right product or support is the lease effective way to succeed. Studies show nearly all cold turkey quit attempts (97 percent) fail."
Now for the rest of the story. All "real-world" quitting surveys continue to find that, outside of clinical studies, which were allegedly double-blind, quitters buying and using replacement nicotine products such as the gum, patch and lozenge have yet to perform better than those quitting cold turkey.
An extensive review of California quitter survey data was published in the September 11, 2002 issue of the Journal of the American Medical Association (JAMA). It concluded that, "NRT appears no longer effective in increasing long-term successful cessation in California smokers."
Subsequent smoker quitting surveys in Minnesota, London, Quebec, and Maryland continue to confirm the California findings. In no quitter suvery assessing cessation at six months or beyond have those quitting while using NRT products performed better than those quitting entirely on their own.
But recently, replacement nicotine has failed to measure up to marketing hype even in rather intense ongoing support settings. A study entitled "The English smoking treatment services: one-year outcomes" was published in the April 2005 issue of Addiction. It examines chemically validated quit smoking rates after one year for quitters who received one-on-one counseling or group treatment for up to eight weeks.
Table six of the English study compares quitting rates for various quitting methods. It shows that 25.5% of those who employed "willpower" and did not use any pharmaceutical quitting product were still not smoking at one year. In stark contrast, only 15.2% who used NRT were still not smoking at one year.
By almost any measure, the wording of GKS's press release grossly distorts America's quitting picture. First it implies that 5% of all quitters are able to quit for one year while only 3% of cold turkey quitters succeed in doing so. This is a horrible example of just how low pharmaceutical interests will stoop in attempting to obtain market share from America's most productive quitting method.
Those marketing quit smoking products almost invariably attribute the lowest one-year quitting rate they can locate to cold turkey (usually 2 to 3%) - as GSK has done - and then conveniently fail to tell readers the one-year quitting rate for their own product. In fact, almost all objective quitting authorities include cold turkey quitters within the historic 5% one-year quitting rate that we've each heard for decades.
Look closely at the GlaxoSmithKline press release. After assigning 3% to cold turkey, isn't it interesting that GSK never once mentions the one-year quit smoking rate for its nicotine gum, patch or lozenge? GSK isn't stupid. It knows that such openness and honesty would kill its golden nicotine producing goose.
The one-year quitting rate for over-the-counter nicotine products is one of the world's best kept secrets. What we do know is the six-month rate as determined by paid consultants to GlaxoSmithKline, Dr. Hughes and Shiffman. Their March 2003 study, published in Tobacco Control, combined and averaged the results of the seven over-the-counter nicotine patch and gum studies.
The study disclosure states, "Dr Hughes has received speaking and consulting fees and grants from Glaxo-SmithKline ..." "Dr Shiffman serves as a consultant to GlaxoSmithKline Consumer Healthcare (GSKCH) on an exclusive basis regarding matters relating to smoking cessation." Dr. Hughes and Shiffman found that, on average, just 7% of over-the-counter (OTC) nicotine patch and gum study participants were still not smoking at six months.
If only 7% of NRT users are still not smoking at six months then how many succeed in quitting for one year? The only way to make an educated guess is to look at older non-OTC clinical studies.
In those studies it wasn't at all unusual to see 40% of successful six-month NRT quitters relapse to smoking between 6 months and one year. If a 40% relapse rate is applied to the 7% six-month rate then only 4.2% of over-the-counter patch and gum quitters are still not smoking at one year.
If the 4.2% figure is anywhere near accurate, imagine the average smoker's reaction to a GSK press release asserting that 4.2% of NRT quitters quit smoking for one year versus only 3% of cold turkey quitters. It would suggest why GSK would not want cold turkey quitters to be part of the 5% one-year quitting rate noted in its release.
It's beyond time for the entire pharmaceutical industry to begin leveling with smokers about their odds while using over-the-counter nicotine quitting products. They need to come clean about two other important factors. It needs to be widely known that unlike repeat attempts at cold turkey quitting, a quitter's odds of success actually decline with repeat NRT use. They also should stop telling quitters that double-blind clinical studies have proven that NRT "doubles their chances" when they know that the studies were not blind as claimed.
If you have already tried quitting with the nicotine patch once then what are your odds during a second or subsequent patch attempt? Only two nicotine patch studies have ever looked at what the NRT industry terms "recycling." A 1993 study (Tonnesen) found that 0% of second-time patch users succeeded in quitting for 6 months and a 1995 study (Gourlay) reported a 1.6% six-month quitting rate. But why?
Almost all online quit smoking authorities carry statements such as this one from the U.S. Centers for Disease Control. "Nicotine is a very addictive drug, and usually people make two or three tries, or more, before they successfully quit. Each time you try to quit, you can learn what works for you and what situations are problematic."
The key lesson eventually learned is the amazing power of one puff of nicotine to completely destroy a quitting attempt. It's called the "Law of Addiction." Eventually successful quitters learn to stop treating their dependency as though it were some "nasty little habit" capable of being controlled or manipulated, and start treating it like a true chemical addiction.
But what lesson could possibly be learned from repeat toying with pharmaceutical grade nicotine? The Tonnesen and Gourlay studies suggest "none." It's why even with an extremely low 5% one year quitting rate for uneducated and unsupported cold turkey quitters, an endless river of cold turkey school of hard-quitting-knocks learning leads to ocean of success (90% of all successful quitters), while NRT's river of attempts trickle into insignificance before almost totally evaporating.
It is also time for the pharmaceutical industry to stop and reflect upon whether or not it is false and deceptive advertising to continue to tell smokers that NRT "doubles their chances" when it knows that those odds ratio victories were generated by studies that were not blind as claimed.
The industry knew since the first nicotine gum studies in the early 80s that it had serious study blinding concerns on its hands. Nicotine is a psychoactive chemical producing alert dopamine/adrenaline intoxication. Smokers with any prior quitting history know what it's like to have their expectations of receiving nicotine fulfilled or denied.
Smokers were often coaxed into quitting studies by being promised a 50/50 chance of receiving weeks or months of free replacement nicotine products. Half were randomly assigned to receive an inert placebo and the other half the "Real McCoy" - pharmaceutical grade nicotine. How many minutes would it have taken any experienced quitter to determine whether or not their gum contained nicotine?
Would frustrated and fulfilled expectations have impacted both placebo group drop-out rates and rates for active group members who decided to remain? In many early studies counseling, telephone support and/or group therapy -- interventions with proven effectiveness -- were offered for weeks or sometimes months, when most of the placebo group dropped-out of the study within the first couple of weeks.
A study appearing in the June 2004 edition of Addictive Behaviors identified 73 double-blind placebo controlled NRT trials and found that only 17 had conducted blinding assessments. During assessments participants were asked to surmise whether they had been using a real nicotine delivery device or an empty placebo.
According to the authors, 12 of the 17 studies (71%) reported blinding failures as "subjects accurately judged treatment assignment at a rate significantly above chance."
If true, and NRT studies were not blind as claimed, should the pharmaceutical industry continue to assert that NRT doubles a quitter's chances when there is absolutely no credible evidence that those odds ratio victories reflect anything other than frustrated and fulfilled participant expectations?
If you are among the 7% who previously succeeded in quitting long-term with NRT before later relapsing, and want to give it another shot, then by all means go for it. But if you are among the 14 out of 15 prior patch or gum users who relapsed to smoking within six months then you have every right to know that your odds of success during a second attempt would be vastly worse.
If you want to join the more than 40 million Americans who successfully quit smoking cold turkey, you'd be wise to spend a bit of time reading their insights and mastering the art, science and psychology of ending all nicotine use. Visit WhyQuit.com, the Internet's leading cold turkey quitting resource and make one complete read of Joel's Library, a free online collection of one-hundred short quitting articles on almost every cessation topic imaginable.
Regardless of how any successful nicotine quitter begins their journey to lasting freedom it eventually ends the same for all ... no nicotine just one day at a time, Never Take Another Puff ... Dip, Chew, Patch or Lozenge!
John R. Polito is solely responsible for all of the above content. It was not reviewed by any other person prior to Internet publication. Any error will be promptly corrected upon receipt of an independent research authority indicating that any fact expressed is incorrect.