
Tobacco control integrity champion demands
review of U.S. smoking cessation policy
Boston University School of Public Health Professor Michael Siegel yesterday called for U.S. health officials and independent researchers to reassess the effectiveness of nicotine replacement therapy (NRT) and its role as the cornerstone of U.S. quit smoking policy. His call follows a rare glimpse of blinding integrity assessment results, revealed in a disturbing nicotine patch study by the patch's co-inventor, which disclosed that four times as many participants correctly identified randomized assignment to the placebo patch as incorrectly identified assignment to the nicotine patch.
Professor Michael Siegel, who is also a physician, today serves as the unelected and unappointed head of the tobacco control movement's internal affairs division. His constant watch-dog fact checking and relentless insistence that the movement be driven by science, not profits, financial influence, exaggeration, or revenge, has endeared him to few. It looks like things are about to get worse.
Dr. Siegel is calling for scientific investigation into the use of placebo controls in nicotine replacement therapy (NRT) clinical trials. He wants those conducting the research to not have any pharmaceutical industry financial ties. He calls for new quitting studies that, for the first, force NRT users to compete head-to-head with real cold turkey quitters, not quitters who joined the study seeking free replacement nicotine but instead received a placebo.
Should the Obama Administration give voice to Dr. Siegel's call, U.S. smoking cessation policy could see dramatic change, with pharm industry profits from the sale of NRT taking a serious blow.
Dr. Siegel's call for an investigation of NRT, at his site entitled "The Rest of the Story," opens by stating, In light of yesterday's revelation (post #1; post #2) concerning the failure of the blinding in nicotine replacement therapy (NRT) trials, and also in light of the way in which financial conflicts of interest with pharmaceutical companies have resulted in bias in the reporting of the results of these studies, I think it is time for a re-examination of the effectiveness of NRT and its role as part of a national smoking cessation promotion strategy.
He notes that current U.S. cessation policy (whose latest revision was reviewed by WhyQuit in May 2008) recommends that "NRT or other pharmaceutical agents be used with every smoker who wishes to quit" and is "plagued by a number of serious problems." Dr. Siegel then lays out ten points, containing links to prior blogs, which highlight the problems:
1. The panel making this recommendation was heavily conflicted. Its chair and seven members had financial conflicts of interest with pharmaceutical companies that manufacture smoking cessation drugs.
2. The presentation of information to physicians on drug treatment for smoking cessation has been found to be biased, presumably because of these financial conflicts of interest.
3. The conclusions of a number of the individual studies of NRT therapy appear to be biased, also presumably on account of financial conflicts of interest. See also this post.
4. Reporting of the financial conflicts of interest in smoking cessation drug studies has been inadequate, making it even more difficult to uncover the role of bias in the reporting and review of this literature.
5. The use of NRT therapy during pregnancy has been specifically challenged.
6. Population-based studies indicate that cold turkey cessation, not the use of NRT, is the most effective method for smoking cessation.
7. A number of recent studies indicate that spontaneous quit attempts, usually conducted without the assistance of NRT, are more effective than planned quit attempts which commonly use NRT.
8. Smoking cessation treatment providers have an odd dislike of electronic cigarettes, suggesting that financial conflicts of interest are playing a major role in skewing the thinking on the issue of national smoking cessation strategy.
9. Blinding failure in NRT clinical trials is a serious concern and has not yet been adequately addressed. As a result, the conclusions of the existing literature have been thrown into doubt. See the following posts for more on this issue: post 1; post 2; post 3.
Blinding failure is a serious concern because when subjects enter into a clinical trial with the thought/hope that they are going to receive nicotine replacement and then they realize they are getting a dud, they may well become very disappointed and discouraged right away. Relapse is very likely under such circumstances. This immediately lowers the continuous abstinence rates in the placebo group. There may be some recovery but it is unlikely that this initial effect can be overcome.
10. Failure to compare NRT to cold turkey quitting: In order to credibly claim that NRT is effective, one needs to compare NRT not to placebo, but to cold turkey quitting.
Dr. Siegel concludes by stating, "In light of these 10 problems, I believe that it is time for a serious re-examination of both the effectiveness of nicotine replacement therapy and the role of NRT as part of a national strategy for the promotion of smoking cessation."
Need for Unconflicted Researchers
"Most importantly," he says, "this re-examination needs to be conducted by unconflicted researchers who do not have financial interests in pharmaceutical companies which stand to benefit from the recommended use of nicotine replacement products."
But as Dr. Siegel himself is forced to acknowledge, "the tobacco control field has become so intertwined with pharmaceutical company money -- even its national and international conferences are now sponsored by Big Pharma -- that I see little possibility for such an unconflicted re-examination of this issue to take place.
U.S. health officials assert that smoking is our nation's leading cause of premature death. If true, and almost all U.S. smoking cessation researchers have already been heavily conflicted by pharmaceutical industry financial ties, then how do we obtain the independent research that America can trust? The solution could rest in U.S. health officials using existing NIH research funding to hire fresh, new researchers.
But are senior health officials alarmed enough? Are they troubled by the fact that nearly all real-world quitting method surveys report cold turkey as the winner? Does it disturb them that although nearly all clinical trials conclude that NRT doubles cessation rates, that the U.S. smoking rate has remained almost unchanged since June 2000? Do they appreciate that it was then that the U.S. cessation policy effectively tied health agency hands and forbid them from openly aiding, supporting, counseling and encouraging what, by far, continues to be our nation's most productive quitting method, cold turkey?
Will Kathleen Sebelius, Secretary of the Department of Health and Human Services, demand that national quit smoking policy be driven by science not profits? Will the National Institute of Health demand integrity and common sense in clinical trial research funded by the U.S. government? Will the Agency for Healthcare Research and Quality (AHRQ), the agency responsible for identifying and featuring the most reliable smoking cessation evidence available, end the practice of allowing U.S. cessation policy to authored by expert panels heavily influenced by pharmaceutical industry financial ties?
Will current AHRQ Director Carolyn M. Clancy, M.D., again permit the next panel chairman to also be receiving a $50,000 a year annual endowment funded by the company that sells Nicorette, or has she changed her thinking since President Obama was elected? Will Centers for Disease Control Director Thomas R. Frieden, MD, MPH, soon require the CDC's Office on Smoking and Health (OSH) to ensure that the 80 to 90% of U.S. quitters attempting to quit cold turkey are encouraged, not discouraged, in trusting their natural quitting instincts?
I close with a glaring example of government deception in bashing the nation's most effective quitting method. The only known government quit smoking method survey, conducted by the National Cancer Institute (NCI), was never published. Why? It was not published because its findings are contrary to all government advice on how to quit.
In discussing quitting method options, Page 10 of the NCI's October 2008 how to quit booklet "Clearing the Air" (PDF page 12) contains a section labeled "Cold Turkey." It tells readers, "For some smokers, "going cold turkey" seems like the easiest way to quit: Just stop smoking and tell yourself you'll never light up again. This works for some smokers - usually those with the lowest level of nicotine dependence - but not many. Fewer than 5 percent of smokers can quit this way." "Research shows that most smokers have more success with one of the assisted quit methods discussed below."
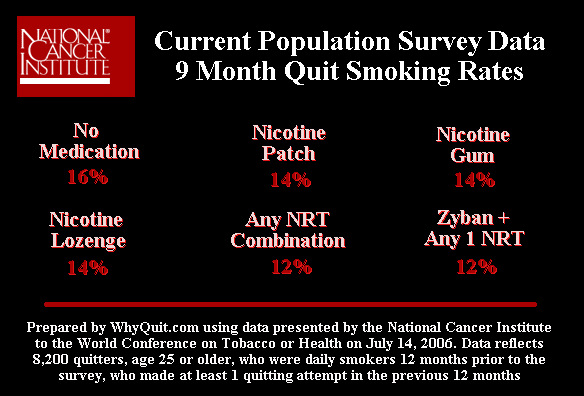
A February 8, 2007 font-page Wall Street Journal article entitled "NICOTINE FIX - Behind Antismoking Policy, Influence of Drug Industry" featured a 2006 NCI quitting method survey that found a 9 month cold turkey quit smoking rate of 16%, more than three times higher than its quitting book, Clearing the Air, asserts. It also shows that those quitting without resort to the nicotine patch, lozenge or Zyban actually did better at six months that those using them.
Will the National Cancer Institute begin giving greater weight to the government's own real-world effectiveness findings or continue to hide government studies while pushing placebo-controlled clinical trial efficacy findings that he knows were not blind? Will the NCI continue its campaign to destroy quitter confidence in our nation's most productive and effective quitting method?
Can science prevail over corporate profits, researcher financial interests, and the group think their placebo-controlled clinical efficacy findings have fostered? Will it? Only time will tell.


