
Is a 14% Chantix success rate worth risking death?
 While nearly all Pfizer Chantix marketing boasts a 44% success rate, a new clinical trial found that only 1 in 7 Chantix quitters were still not smoking at 6 months. This comes as Chantix adverse reaction reports to the FDA surpass a whopping 35,000.
While nearly all Pfizer Chantix marketing boasts a 44% success rate, a new clinical trial found that only 1 in 7 Chantix quitters were still not smoking at 6 months. This comes as Chantix adverse reaction reports to the FDA surpass a whopping 35,000.
A June 7, 2011 study in the journal Nicotine & Tobacco Research found that only 14% of Chantix users were still not smoking at 24 weeks. Headed by University of Vermont physician-professor John Hughes, ironically, it arrives as New England news headlines tell of a Vermont Chantix user's shotgun slaying of his mother.
The Hughes study comes on the heels of a May 19, 2011 report by the Institute for Safe Medication Practices (ISMP) revealing that the FDA has now received a total of 272 reports of completed suicides by Chantix users.
According to the ISMP, Chantix ranks first in reported deaths, more than twice as many as any other monitored drug. Total adverse event reports now exceed 35,000, of which roughly 10,000 were classified as serious, disabling or fatal, with 1,055 serious events being reported during the 3rd quarter of 2010.
So how do we explain the substantial difference between Pfizer's 44% advertised success rate and this study's disturbing 14% finding? One major difference is that Pfizer's 44% rate declares quitting victory at 12 weeks, the end of treatment, a day when many study participants were still under varenicline's influence (24 hour elimination half-life).
The Hughes paper's 14% figure is a six-month rate following 8 weeks of treatment. It was established three months after most users had stopped using Chantix, and had adjusted to living with natural dopamine pathway stimulation.
The 44% advertised rate reflects continuous cessation (zero smoking lapses) and was generated in a July 2006 drug approval study published in the Journal of the American Medication Association (Gonzales 2006 - free full text).
There, the more comparable six-month (24-week) 7-day point prevalence rate (no smoking within the prior 7 days of the smoking assessment point) was 33.5 percent. But that's a rate 239% higher than Hughes' six-month (24-week) 7-day point prevalence rate of 14%.
The level of formal counseling and support provided in generating the 44% advertised rate was more than twice the level seen in the Hughes study. Pfizer's 2006 drug approval studies provided participants with 19 counseling/support sessions by the 24-week mark. The Hughes study involved just 8 provider contacts: 4 counseling sessions and 4 follow-up telephone calls seeking data.
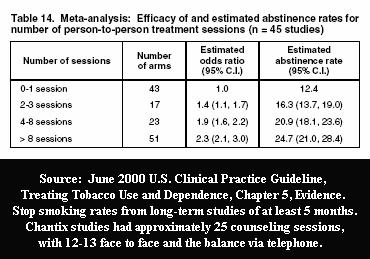 What's concerning is that according to the June, 2000 U.S. Guideline for Treating Tobacco Use and Dependence, Pfizer knew when it designed the original Chantix studies that counseling and support program contacts are highly effective at helping prevent relapse to smoking.
What's concerning is that according to the June, 2000 U.S. Guideline for Treating Tobacco Use and Dependence, Pfizer knew when it designed the original Chantix studies that counseling and support program contacts are highly effective at helping prevent relapse to smoking.
According to the Guideline, quitting programs involving 4 to 8 sessions generate an average 24 week quit smoking rate of 20.9 percent. What's not yet known is whether the Hughes study's dismal 14% rate suggests that Chantix is worthless as a quitting aid.
Surprisingly, the FDA allowed Pfizer to bring Chantix to market without any study showing quitting rates when unaccompanied by formal counseling and support, the manner used by the vast majority of smokers trying to quit.
According to the U.S. Guideline, the record levels of counseling and support seen in Chantix's 2006 drug approval trials - up to 25 sessions, each lasting up to ten minutes - could account for nearly all of the 44% quitting rate boasted by Pfizer.
Still a bit high on the number of provider contacts, the Hughes trial is the closest yet to mimicking real-world use conditions. Real-world users often are not highly motivated, but decide to give a new quitting product a try following recommendation by a physician, marketing, family or friend.
The level of participant cessation motivation may have contributed to the Hughes 14% rate. Study inclusion criteria required all participants to state that they eventually wanted to stop smoking but that they had no planned intention to quit within the next month.
Smokers were recruited to the study via advertising which stated, "Smokers, not quite ready to quit? We are testing a new medication to help you reduce and control your smoking and be less addicted. Compensation up to $75 provided. This is a study conducted by the University of Vermont."
The Hughes study's average participant smoked 19 cigarettes per day, was 42 years old, started smoking about age 16, and rated their addiction at 8.5 on a 10 scale.
What Pfizer marketing continues to fail to adequately advise smokers is that unless Chantix use is accompanied by effective and ongoing counseling or support that the user's odds of success are not good. In fact, they're horrible. What Pfizer fails to make clear is that its 44% advertised rate was generated by participants who looked forward to receiving professional face-to-face counseling during every week of treatment.
The FDA has known for decades that smokers generally do not like attending or participating in quitting programs. In the future, the FDA should not approve any quitting product without a stand-alone study requiring the product to stand on its own two feet, unsupported by counseling or support. Sadly, it's a study that, if the pharmaceutical industry has its way, as here, will never be conducted.
Ten thousand serious adverse events, a six-month rate of just 14 percent, and no statistically significant advantage in generating successful quitters by six months or one year when pitted against the nicotine patch (see Aubin 2008). Before the death toll climbs higher, it's time for the FDA to engage in serious and immediate risk-benefit analysis as to whether or not Chantix should remain on the market.
John R. Polito is solely responsible for the content of this article. Any factual error will be immediately corrected upon receipt of credible authority in support of the writer's contention. E-mail comments to john@whyquit.com

