FDA knew stop smoking product clinical trials not science-based
Hundreds of placebo-controlled NRT & Chantix studies not blind as claimed
February 2, 2019 John R. Polito
 Faced with a teen vaping/juuling epidemic, the US Food & Drug Administration (FDA) announced that it was considering "the potential role of drug therapies to support youth e-cigarette cessation" and invited public comment. In response, the below comments were electronically submitted to the FDA on January 31, 2019 (CTN: 1k3-97zu-27gy), with a hard copy mailed February 1.
Faced with a teen vaping/juuling epidemic, the US Food & Drug Administration (FDA) announced that it was considering "the potential role of drug therapies to support youth e-cigarette cessation" and invited public comment. In response, the below comments were electronically submitted to the FDA on January 31, 2019 (CTN: 1k3-97zu-27gy), with a hard copy mailed February 1.
From: John R. Polito, Nicotine Cessation Educator
Re: Comment Submission to: Eliminating Youth Electronic Cigarette and Other Tobacco Product Use: The Role for Drug Therapies; Public Hearing; Request for Comments: Docket ID: FDA-2018-N-3952, Agency: Food and Drug Administration (FDA), Parent Agency: Department of Health and Human Services (HHS): Comment Tracking Number: 1k3-97zu-27gy
Title: How can the FDA be expected to be honest with juul addicted teens about quitting when it has yet to be honest with smokers?
Dear Commissioner Gottlieb and FDA:
While the following comments are highly critical of HHS and the FDA’s history in undercutting U.S. smoking cessation, I hope you will read and reflect upon them. For if we refuse to examine where we started and where things now stand, what hope is there in making correct decisions in how best to aid the nicotine dependent adolescent?
Where Things Stand
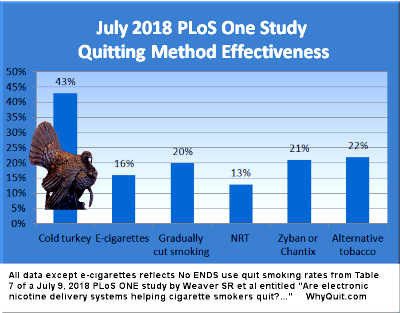
After thirty-five years of telling smokers that pharma’s nicotine is medicinal and its use therapeutic, data from the most comprehensive population-level quit smoking study ever shouts that - as with all nicotine weaning schemes - use of replacement nicotine can double your chances of relapse and failure.[1] It’s a real-world effectiveness finding mirrored in earlier studies.[2]
Yes, I understand the industry’s confounding by indication argument, that smokers who struggle to stop are more likely to seek and use approved products, while those who find quitting easier are less likely to do so. But if accurate, where could such confounding possibly be greater than in clinical trials dangling free approved products as study recruiting bait?
Population level findings reflect the totality of cessation. In that GlaxoSmithKline and Pfizer quitting product marketing never discriminates in the dependency level of the mind it strikes, why should population data require adjustment?
While the FDA’s cessation focus is primarily in regulating and approving cessation products, Section 918 of Family Smoking Prevention and Tobacco Control Act also expressly charged the FDA with submitting to Congress a report on how best to promote and encourage “non-nicotine-based treatments,” with an aim of achieving “total abstinence of tobacco use.”
Despite up to 75-80 percent of ex-smokers quitting cold turkey,[3] the FDA chose to read Section 918 so as to totally abrogate responsibility for investigating, understanding, promoting or encouraging successful abrupt nicotine cessation.
The FDA’s response to mounting evidence that approved product clinical efficacy and real-world effectiveness are diametric opposites has been to stick its head in the sand.
Now, the FDA appears poised to encourage Nicorette, Nicoderm CQ, Habitrol and Zyban use by e-cigarette dependent youth.
What’s needed are more studies like Weaver 2018 and Doran 2005,[1] studies painting full and complete population-level quitting method productivity and effectiveness pictures. And not just for adults but for adolescents too.
Unfortunately, it won’t happen. Why so negative? Because I’ve lost hope. Because I’ve watched the U.S. Department of Health and Human Services (HHS) keep hidden the only known government produced quitting methods survey (Hartman 2006 NCI). Because HHS’s 944 page 2018 version of the U.S. Population Assessment of Tobacco and Health (PATH) survey doesn’t ask a single question about abrupt nicotine cessation, cold turkey or unassisted quitting, while asking multiple questions about every approved quitting product.
Because, as harsh as this sounds, HHS continues to deceive smokers in telling them that “not many” are able to quit cold turkey (Clearing the Air, 2006 NCI – see booklet page 10). Because, year after year HHS intentionally keeps smokers, quitters and teens in darkness as to the most important quitting question of all, how the vast majority of smokers successfully arrest their chemical dependence upon nicotine and become comfortable ex-users (see, thousands and thousands of HHS “smoking” documents).
Why would HHS oppose creating and sharing full, factual and robust U.S. nicotine, tobacco and smoking cessation pictures evidencing what’s effective and producing and what isn’t? A number of reasons.
Most obvious is the potential for truth to undermine the FDA’s medicinization of smoking, tobacco and nicotine cessation.
The FDA, CDC and NCI have known since at least the Pierce 2002 JAMA study that they’d totally invested their credibility and reputations in pharma’s nicotine, and that “since becoming available over the counter, NRT appears no longer effective in increasing long-term successful cessation in California smokers.”
Instead of putting down the shovel, or after Mooney 2004, launching an investigation into whether it was ever possible to blind experienced quitters as to recognition of the presence or absence of their withdrawal syndrome, the FDA, CDC and NCI have continued digging.
How We Got Here
What’s needed is long overdue light on the fact that the FDA was alerted as to horrific blinding concerns on June 22, 1983, prior to approving nicotine gum on January 13, 1984, and has since knowingly aided in keeping it hidden.
As this review will evidence, while placebo is the gold standard in most research, in smoking cessation it has been license to steal.
Asked to apply the FDA’s safe and effective standards to a natural insecticide, the primary reason the 2009 Family Smoking Prevention and Tobacco Control Act (FSPTCA) granted the FDA and not the Federal Trade Commission jurisdiction over tobacco and nicotine is that 25 years earlier, on June 22, 1983, the FDA’s Drug Abuse Advisory Committee erred in declaring that adequate and well-controlled studies evidence efficacy in nicotine gum increasing the likelihood of smoking cessation among participants receiving counseling.
The Advisory Committee asked the most critical questions that Wednesday morning in June. What it failed to reflect upon was that, prior to being rushed to judgment in finding Nicorette efficacious, its concerns went unaddressed.
Nicotine Psychoactive
Dr. Jasinski reviewed with the Committee the fact that nicotine is a psychoactive drug. "I define it when given versus placebo under various circumstances [it has] the ability to alter mood, feeling states, thinking, and perception.” [Page 98]
“Delivered intravenously or inhaled, it is psychoactive and physiologically active; that is, people can tell it from placebo and they can discriminate the content of cigarettes when you smoke them under certain characteristic ways, and they can discriminate among different nicotine contents of cigarettes and they can discriminate among boluses of saline and various doses of nicotine given intravenously.” [Page 98]
Dr. Jasinski went on to declare that nicotine gum “is psychoactive. Subjects can discriminate.” [Page 105].
At that moment, with the committee openly declaring that the active group was aware they were receiving nicotine, while the placebo group could sense they were not, and zero evidence to the contrary, all studies reviewed should have been declared to not be blind, compromised by assignment awareness, measuring expectations, inflated or diminished by the recognized value of counseling or the importance of study contacts, and a motion made to adjourn. But that didn’t happen.
Earlier, the committee had sampled the placebo gum that was used in the Christen dental study. [ Page 48]
"Nasty" Placebos
“One other concern I had was that neither the protocol nor the study report submitted by the sponsor indicated whether or not there was a discernible difference between the taste of Nicorette and placebo chewing gums,” said Dr. Marticello. “This is a factor that could possibly have affected the blindedness of the study. I guess some individuals have tasted the placebo already, although not the Nicorette.” [Page 87]
“Are the placebos indistinguishable from the 2-milligram-containing nicotine gums? asked Dr. Goodwin earlier. “Can you tell the difference between them and the active drug?” [Page 30]
“I have not chewed this placebo before, and this is quite distinguishable,” replied Dr. Jones. “I find this not unpleasant.” [Page 30 ]
What about the active group when chewing the 2mg gum?
Dr. Jones had previously sampled the nicotine gum. He stated that it has a “different taste, plus you do get effects - I would call it side effects - you get the effects of nicotine, at least I, as a nonsmoker, do.” [Page 30]
“I am pretty sure the person can, by pharmacologic effects detect,” replied Dr. Martz. “But the taste is - - we put as much nastiness in this as we could get.” [Page 30]
According to studies sharing how each study’s active and placebo gum, patches or lozenges were obtained, they were generally supplied by the pharmaceutical industry.
More than 200 placebo-controlled NRT trials, and with each passing study the industry developed greater insight and awareness as to which placebo formulations generated newsworthy findings and which did not.
Active Placebos
Dr. Reese T. Jones was troubled that the other primary study offered in support of Nicorette approval, the Russell study, had used an active placebo containing nicotine.
It was explained that while the active gum was a 2mg commercial preparation, “the placebo was the 1-milligram unbuffered gum, and the lack of buffer meant that even the lower dose of nicotine was much less well absorbed.” [Page 42]
“How much nicotine should be in it?” he asked. “And since we don't know the bioavailability at that dose at those levels in the unbuffered form, really, I think Dr. Russell's data are reassuring, but they are certainly not the sort of data I think we would demand if this were almost any other drug, treating almost anything other than tobacco dependence.” [Page 106]
Think about what Dr. Jones was saying. Because we’re dealing with smoking and patients already addicted to nicotine, that the science can be sloppy, that guessing is allowed, that we don’t need to know the precise consequences of adding 1mg of unbuffered nicotine to placebos.
It’s important because use of active placebos has been acknowledged in a number of nicotine patch studies too (1996, 1997, 2002).
Were active placebos spiked with just enough nicotine to keep users in the tease and throws of withdrawal: not delivering enough to satisfy cravings, nor allowing them to get clean, begin re-sensitizing, and move beyond peak withdrawal within 3 days?
How widespread was use of active placebos? We have no idea. It would appear that after hearing all this, the FDA doesn’t care as there have been no known studies, no investigation, and no industry use claims have been forthcoming.
The unanswered blinding integrity assessment question was asked by Dr. Paul. “How well did your patients … predict whether they were on placebo, how accurately could they predict whether they were on placebo, or did you do any of those kids of experiments? Because the differences, although they are significant, maybe twice in terms of abstinence, are still relatively small, and I am really concerned that these are real drug-placebo differences, drug/inactive placebo differences versus active placebo, and I am curious as to whether patients could retrospectively reliably tell.” [Page 61]
Dr. Russell’s response? “We did not actually tell patients they were receiving the placebo.” “We said we were trying out nicotine, would they enter for a trial of nicotine-containing chewing gum.” [Page 61]
What the committee totally missed was the overarching fact that experienced quitters had become experts at recognizing their withdrawal syndrome, and that these discrimination experts would become keenly aware of their assignment within 24 to 48 hours of quitting (peak withdrawal).
Prior to the committee voting, Dr. Leber stated, “If you were to conclude that [nicotine gum] is a horror for the public health, by all means say so and tell us not to proceed, because we don't want to make any mistakes.” [Page 113]
In response, Dr. Paul, like Dr. Jones, wanted to know if there were any data from the 1.2 million people who had already used nicotine gum in nations that had already approved it. [Page 114]
Nicorette’s manufacturer then and there failed to provide the FDA Advisory Committee with basic population effectiveness findings, critical performance data that’s been kept hidden every year since.
NRT, Zyban and Chantix Trials Not Blind as Claimed
A line of studies by KA Perkins teach us that smokers and nicotine-naive participants can be trained and conditioned to reliably distinguish varying doses of nicotine.
Mooney 1984 examined the few clinical trials that had conducted extremely poor quality blinding integrity assessments. It found that placebo-controlled clinical trials were generally not blind as claimed in that participants could correctly declare assignment at rates significantly above chance.
Mooney concluded by encouraging researchers to conduct quality integrity assessments and adjustments if needed, and warned of the consequences of failing to do so.
“To determine the prevalence of failure, clinical trials of NRT should uniformly test the integrity of study blinds. Moreover, if blindness failure is observed, subsequent efforts should be made to determine if blindness failure is related to study outcome and, if so, to provide an estimate of treatment outcome adjusted for blindness bias. Without these methods and analyses, the validity of NRT clinical trial results could be questioned.”
Amazingly, only two post-Mooney studies included blinding integrity assessments, and only one made adjustments. In Dar 2005, the fact that 3.3 times as many placebo users were able to correctly identify their randomized assignment as declared wrong altered the study’s outcome.
In Rose 2009, within one week of quitting, 4 times as many placebo patch users were able to correctly declare their assignment as declared wrong ("of 165 subjects receiving placebo patches, 27 believed they had received active patches, 112 believed they had not, and 26 were unsure").
If more than 50 million “double your chances” U.S. nicotine gum quitting attempts have been made since 1984, where are the success stories? After 29 years as the FDA’s cornerstone of smoking cessation, and billions spent on marketing, a July 2013 Gallup Poll found that only 1 percent of U.S. quitters credited nicotine gum for their success.
Talk about the tail wagging the dog and HHS’s shocking “cold turkey” cover-up, the 2013 Gallup Poll found that 92% of successful ex-smokers did not credit NRT, Zyban or Chantix. Nearly all had succeeded without it.
Sadly, it appears that the correct answer to Dr. Leber’s closing nicotine gum public health horror question is a resounding “yes, it is.” Under real-world use conditions, as suggested by nearly all population-level quitting method data, OTC NRT undercuts successful cessation. At what price in terms of delayed cessation and lives lost?
FDA Allowing Marketing to Run Wild
Brought up by Dr. Jones, is it coincidence that the final concern presented to the 1983 FDA Advisory Committee addresses what I’ve tried to get HHS to focus upon for almost two decades, allowing the tail to lie while wagging the dog. From “double your chances” quitting product marketing to Pfizer’s new Chantix “slow turkey” “keep smoking” campaign and its “I’m tough-guy Ray and I couldn’t quit cold turkey” campaign, marketing has been allowed to bash, trash and undermine confidence in our nation’s undefeated population-level production and effectiveness champion, cold turkey.
As Dr. Jones put it, "Most smokers who want to stop smoking stop smoking without any particular professional intervention, is my guess. I have seen good data on this and perhaps Dr. Russell or someone else may have some data on how many smokers are able to stop without any intervention."
"What the availability of this will do, the gum will do, is more incline people to resort to pharmacotherapy when odds are they don't need pharmacotherapy." "[I]t is a consideration in terms of perhaps demanding the best evidence of efficacy that we can." [Pages 122-123]
Pharma Influence Controlling U.S. Cessation Policy and HHS
What Dr. Jones couldn’t then predict was that the cessation pharmaceutical industry would create a platoon of skilled and well-compensated PhD principal investigators, some of whom HHS would allow to play major roles in influencing and authoring official U.S. cessation policy.
What the committee couldn’t then know was that seventeen years later, in June 2000, under the guidance of a chairman then sitting in a million dollar university chair endowed by Glaxo-Wellcome (Nicorette’s maker), with 11 of 18 panel members having declared pharmaceutical industry financial ties, that HHS support for the method that had produced nearly all successful U.S. ex-smokers was about to be permanently banned.
How could Dr. Jones possibly anticipate that, mirroring Glaxo-Wellcome’s written endowed chair agreement, that the U.S. 2000 Guideline would require that, henceforth, every smoking patient be urged to quit by using pharmacotherapy (compare Guideline recommendation 7 on page iv)?
Today, the pharmaceutical industry pays 75 percent of the FDA’s drug approval budget, while the FDA’s tobacco program is 100 percent funded by tobacco product user fees, 96.33 percent of which are indirectly paid by chemically enslaved cigarette smokers.
Both smokers and juuling teens deserve the truth. But, respectfully, that has not happened with Mitch Zeller as director of the FDA’s Tobacco Products Office since March 2013.
Should we close our minds to the reality that GlaxoSmithKline stands to benefit handsomely by recommending NRT use by addicted teens, or that from 2002 to 2013 Zeller was a senior vice president for Pinney Associates, GlaxoSmithKline's exclusive quitting products marketing corporation?
Zeller’s September 2012 FDA directorship audition, his continuum of risk “tobacco endgame” analysis, doesn’t mention nicotine dependency recovery. Instead, to GlaxoSmithKline’s delight, Zeller’s endgame involves long-term use of “the current generation of medicinal nicotine products such as gum, patches and lozenges.”
Ask yourself, does the phrase “your chances” suggest that we are referencing real world effectiveness findings or clinical trial efficacy victories over placebo users who wanted the real thing but didn’t get it?
If the former, a December 2017 FDA page, created under Zeller’s watch, openly feeds smokers and juuling teens the falsehood that “using FDA-approved cessation medicine can double your chance of quitting successfully.”
As for Zeller’s endgame NRT thinking, the FDA’s new page also tells smokers that “the FDA recognizes that some people may need to use these products longer to stay smoke-free. Talk to your health care provider to determine the best course of treatment for you.”
Now, Pharma Wants Our Children Too
Vastly too pharma friendly and trusting, an NRT approval house of cards built upon nasty tasting placebos which did nothing to diminish the experienced quitter’s recognition of peak withdrawal, or in the case of active placebo use, possibly teasing and torturing the control group into relapse, the FDA stands poised to extend the most deadly science sham in history to juuling children and teens.
I beg the FDA to resist the urge.
What our nicotine dependent young need is honest and accurate recovery info, not more nicotine.
They need to hear their health officials tell them the truth about nicotine and addiction, that they may begin losing the autonomy to turn and walk away after vaping just a time or two.
Children and teenagers need an appreciation as to what nicotine addiction is and means, that like them feeling urges or cravings for food two or three times daily, that those same brain dopamine pathways can quickly begin to behave as if nicotine is food.
Have students try to imagine their brain generating urges and craves for more nicotine 5, 10, 15 or eventually even 20 times daily, every day, from waking up until bed, for the rest of their lives. Get them to imagine difficulty concentrating in class because there is a war in their brain as they try fighting off urges.
Students need to appreciate that nicotine addiction is REAL drug addiction, a brain wanting disorder and true mental illness that’s as real and permanent as alcoholism.
While they may fully and comfortably arrest their chemical dependence they cannot kill or cure it. After quitting, just one puff, dip, vape or chew and it won’t be long before they find their brain wanting, plotting to obtain or even begging for more.
If the FDA really wants to promote successful adolescent quitting, it needs a correct and accurate answer to the most fundamental quitting question of all. What is the key to successful abrupt nicotine cessation?
I submit that the answer is the exact opposite of the lapse-relapse advice currently shared by HHS at SmokeFree.gov.
Imagine the insanity of teaching recovering alcoholics that "Slipping and having a [drink] or even going back to [drinking] for a little while is not failing. It is normal." "A slip doesn't make you a [drinker] again."
Instead of inviting, encouraging and promoting relapse, nicotine dependent adolescents and adults need awareness that just one puff and up to half of their brain dopamine pathways would become occupied by nicotine.
As found by the Brandon 1990 study, nearly all who “tasted” a cigarette relapsed (88%). "The high rate of return to regular smoking once a cigarette is tasted suggests that the distinction between an initial lapse and full relapse may be unnecessary."
The Garvey 1992 study followed 235 adult smokers for one full year after attempting to quit. It found that, "Those who smoked any cigarettes at all in the post-cessation period (i.e. lapsed) had a 95% probability of resuming their regular pattern of smoking subsequently."
All articles and videos of Joel Spitzer, America’s most studied nicotine cessation educator, end the same. Joel reminds them of the one rule that if followed provides 100 percent odds of success, to never take another puff.
Joel’s Library is home to Spitzer’s almost 500 video lessons and more than a hundred articles on almost every abrupt nicotine cessation topic imaginable. In that HHS has never had a problem mentioning approved quitting products by brand name (as it does at the bottom of this comment invitation), in the spirit of affirmative action, I encourage the FDA to begin referencing Joel’s Library for those seeking info on abrupt nicotine cessation.
In closing, the FDA is nearly as trapped and dependent upon sham placebo-controlled quitting product findings as any student is upon juuling. It has two options. It can continue digging or at last demand full and complete real-world quitting pictures, while beginning its own blinding integrity investigation.
I sincerely hope it chooses the latter.
Respectfully,
John R. Polito, JD
Nicotine Cessation Educator
References:
[1] Weaver SR, Huang J, Pechacek TF, Heath JW, Ashley DL, Eriksen MP. Are electronic nicotine delivery systems helping cigarette smokers quit? Evidence from a prospective cohort study of U.S. adult smokers, 2015–2016. 2018 PLoS ONE 13(7): e0198047. https://pubmed.ncbi.nlm.nih.gov/29985948/.
[2] Doran CM, Valenti L, Robinson M, Britt H, Mattick RP. Smoking status of Australian general practice patients and their attempts to quit. Addict Behav. 2006 May;31(5):758-66. Epub 2005 Aug 31. https://pubmed.ncbi.nlm.nih.gov/16137834/ ; also see Twyman L, Bonevski B, Paul C, Bryant J, West R, Siahpush M, D'este C, Oldmeadow C, Palazzi K. What factors are associated with abstinence amongst socioeconomically disadvantaged smokers? A cross-sectional survey of use of cessation aids and quitting approach. Drug Alcohol Rev. 2018 Feb;37(2):170-179. doi: 10.1111/dar.12561. Epub 2017 Jun 14. https://pubmed.ncbi.nlm.nih.gov/28616900/ ; and Ferguson J, Bauld L, Chesterman J and Judge K. The English smoking treatment services: one-year outcomes. Addiction 100 (Suppl. 2), 59-69 2005, see Table 6 https://whyquit.com/studies/2005_UK_NHS_1_Year_Ferguson.pdf
[3] Benmarhnia T, Pierce JP, Leas E, White MM, Strong DR, Noble ML, Trinidad DR. Can E-Cigarettes and Pharmaceutical Aids Increase Smoking Cessation and Reduce Cigarette Consumption? Findings From a Nationally Representative Cohort of American Smokers, Am J Epidemiol. 2018 Nov 1;187(11):2397-2404. https://academic.oup.com/aje/article/187/11/2397/5046037Tarik https://pubmed.ncbi.nlm.nih.gov/29955810/; also see Doran CM, Valenti L, Robinson M, Britt H, Mattick RP. Smoking status of Australian general practice patients and their attempts to quit. Addict Behav. 2006 May;31(5):758-66. Epub 2005 Aug 31. https://pubmed.ncbi.nlm.nih.gov/16137834/.
I, John R. Polito, am fully and solely responsible for the content of this article. Any factual error will be promptly corrected upon notice emailed to john@whyquit.com