UW-CTRI's holiday health media manipulation
The University of Wisconsin's Center for Tobacco Research and Intervention (UW-CTRI) seeks to influence what health journalists and the media write and say about the best way to quit smoking this New Years. Will it succeed? Will health news articles tell smokers to spend twice as much on quitting products as last year? Probably.
 New Year's is by far the biggest stop smoking day of the year. Although we expect pharmaceutical industry holiday season creativity in marketing quitting products, this year the University of Wisconsin (UW) is leading the charge. Recipient of millions of dollars in industry funding, it's payback time and media manipulation is the order of the day.
New Year's is by far the biggest stop smoking day of the year. Although we expect pharmaceutical industry holiday season creativity in marketing quitting products, this year the University of Wisconsin (UW) is leading the charge. Recipient of millions of dollars in industry funding, it's payback time and media manipulation is the order of the day.
The opening line of a December 14 press release by the UW's Center for Tobacco Research and Intervention (UW-CTRI) reads in part, "smokers trying to quit smoking for the holidays have the best chance for success if they take the nicotine lozenge in combination with either bupropion (a pill) or the nicotine patch."
It's the one line GlaxoSmithKline -- maker of the Commit nicotine lozenge, Nicoderm nicotine patch, and Zyban (bupropion) -- hopes health journalists will seize upon in writing this year's batch of "how to quit smoking" articles.
The press release briefly reviews a UW-CTRI study published in the December 14 issue of Archives of Internal Medicine entitled, "Comparative Effectiveness of 5 Smoking Cessation Pharmacotherapies in Primary Care Clinics." Primary care patients motivated to quit smoking were randomized to one of five quitting product groups while also receiving counseling via a telephone quit line.
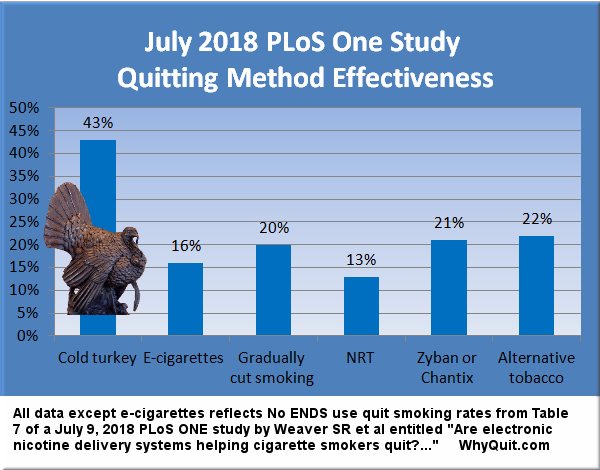
The study and press release boast six-month quitting rates of 29.9% in a group that combined use of both the nicotine lozenge and bupropion, 26.9% in a group using both the nicotine lozenge and patch, 19.9% in a group using the nicotine lozenge alone, 17.7% in a group using the patch alone, and 16.8% among those using bupropion alone.
UW-CTRI and GlaxoSmithKline also hope health journalists include the press release zinger that, "the clear message here is that combining the lozenge with the nicotine patch or bupropion gives smokers the best chance to quit." But is it true?
Study ignores nicotine dependency recovery
What the UW-CTRI press release does not tell journalists is that neither this study nor its November 2009 clinical companion presents any evidence that any study participant actually broke nicotine's grip upon their mind and life. None. Imagine pronouncing those using multiple avenues to stimulate brain dopamine pathways as having been most successful, when blood, saliva and urine were not examined to determine if stimulation by quitting products actually ended.
What percentage of this study's successful quitters remain hooked on nicotine lozenges today? Doesn't informed consent scream that smokers be told?
What we do know is that a 2003 study found that up to 7% of nicotine gum quitters and 37% of all current gum users remain persistent long-term users for at least 6 months, twice as long as the use period approved by the FDA.
Combination treatment clearly has potential to generate the highest blood serum nicotine levels ever documented in quitting studies. It is highly irresponsible for the University of Wisconsin to strongly advocate combination quitting product use while ignoring evaluation of chronic long-term chemical dependency upon NRT and/or Zyban.
Study claims ignore counseling's effectiveness
"The clear message here is that combining the lozenge with the nicotine patch or bupropion gives smokers the best chance to quit." Again, is it true? Were any smokers actually able to arrest their chemical dependency upon nicotine? Additionally, is this "clear message" honest when counseling, and not combination therapy, may account for nearly all of the differences seen?
There is consensus among experts that counseling and support are highly effective at helping smokers quit. The focus and intensity of abrupt nicotine cessation (cold turkey) counseling takes aim at getting smokers through the first three days and peak withdrawal. Pharm product counseling's initial objective is successful dependency transfer to a chemical that stimulates brain dopamine pathways.
While successful cold turkey quitting benefits from daily front-end counseling, the closer cessation pharmaceutical products mimic dopamine levels generated by smoking, the longer the user remains cigarette free and able to benefit from ongoing counseling. Obviously, participants have no need for counseling once they relapse to smoking.
This study arranged telephone counseling through the Wisconsin Tobacco Quit Line (WTQL). Although told that the percentage of each quitting product group actually speaking with WTQL counselors was similar (a low of 35% for bupropion group, to a high of 46% for the bupropion plus nicotine lozenge group), the study fails to disclose the total amount of counseling time received by each group, or the total amount of counseling time received by successful quitters within each group.
Instead, readers are told:
"These results showed that there was not a linear increase in abstinence rates with more minutes of counseling but, instead, users with fewer than 90 minutes of counseling (n=316 had an abstinence rate of 19.6% that was nearly the same as the rate for nonusers of the WTQL (n=801; abstinence rate, 19.5%. In contrast, WTQL users who had more than 90 minutes of counseling had a 6-month abstinence rate of 35.8%."
Shouldn't the "clear message" and big news from this study have been the tremendous value of more than 90 minutes of counseling?
The UW-CTRI press release does not once mention that receiving more than 90 minutes of counseling generated a 36% success rate. Why? Is UW-CTRI's primary concern to help GlaxoSmithKline make money or to help smokers quit?
Press release hides how "real" ex-smokers succeed
UW-CTRI wants this study to be known as a "real-world" effectiveness evaluation. Frankly, it has very little to do with how smokers quit under "real-world" conditions.
How many "real-world" smokers are randomly assigned to one of five quitting groups, given free quitting products, and receive an unsolicited telephone call from a telephone quit line? None. Out here in the real-world the vast majority of all successful quitters do not engage in weeks or months of expensive nicotine weaning. They quit cold turkey.
In stark contrast to UW-CTRI's artificial manipulation and control, a true "real-world" study was published in the April 2006 edition of Addictive Behaviors. It was a hands-off study that simply followed smoking patients of Australian primary care physicians and reported on their quitting attempts, the methods they used and their outcomes.
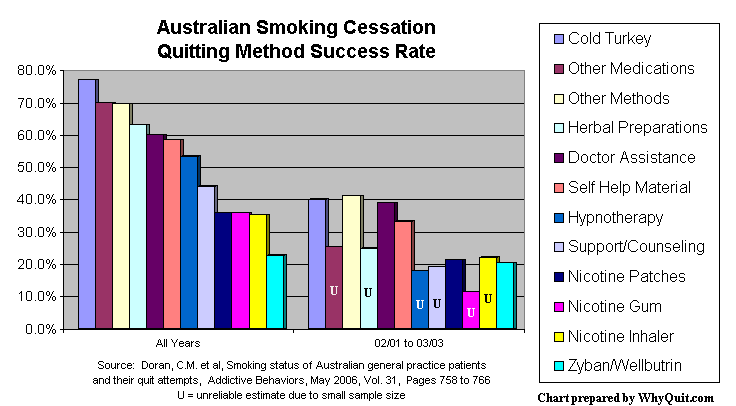
It found that the success rate for cold turkey quitters was twice as high as the rates for those using the nicotine patch, nicotine gum, nicotine inhaler or Zyban (bupropion). Even more impressive, it found that cold turkey quitters accounted for 1,942 of 2,207 former smokers, a whopping 88% of all success stories.
UW-CTRI refuses to document real patient quitting outcomes of Wisconsin primary care physicians. Why? Because even in Wisconsin cold turkey is king and there would be nothing to sell.
UW-CTRI has already played a key role in redefining "quitting." By its definition we should totally ignore chemical dependency upon nicotine and instead focus only upon success in ending use of just one form of nicotine delivery, the cigarette.
Now it seeks to redefine "real-world" quitting, in asking us to ignore and hide how real-world quitters actually succeed, and how quitting rates among those using quitting products are almost always lower than rates achieved by those quitting without them.
UW hides financial conflicts
Amazingly, the University of Wisconsin press release does not alert media to the authors' pharmaceutical industry financial ties. It should. The study's financial disclosure states:
"Dr Smith has received research support from Elan Corporation plc. Dr Jorenby has received research support from Pfizer Inc, Sanofi-Synthelabo, and Nabi Biopharmaceuticals and has received consulting fees from Nabi Biopharmaceuticals. Dr Fiore has received honoraria from Pfizer Inc and has served as an investigator on research studies at the University of Wisconsin that were funded by Pfizer Inc, Sanofi-Synthelabo, and Nabi Biopharmaceuticals. In 1998, the University of Wisconsin (UW) appointed Dr Fiore to a named Chair funded by an unrestricted gift to UW from Glaxo Wellcome. Dr Baker has served as an investigator on research projects sponsored by pharmaceutical companies including Sanofi-Synthelabo, Pfizer Inc, and Nabi Biopharmaceuticals."
Will health reporters eventually awaken to researcher study games and industry marketing tactics? Maybe not. It's much easier to simply regurgitate a study press release than to look behind its words to the truth beyond.
I, John R. Polito, am solely responsible for the content of this article. Any errors brought to my attention will be immediately corrected.
Written December 16, 2009 and last updated December 17, 2009