NICOTINE FIX
Behind Antismoking Policy, Influence of Drug Industry
Government Guidelines Don't Push Cold Turkey; Advisers' Company Ties
Wall Street Journal - February 8, 2007, Page A1
by Kevin Helliker
Michael Fiore is in charge of revising federal guidelines on how to get smokers to quit. He also runs an academic research center funded in part by drug companies that make quit-smoking aids, and he personally has received tens of thousands of dollars in speaking and consulting fees from those companies.
Conflict of interest? No, says Dr. Fiore, who has consistently declared that doctors ought to use stop-smoking medicine. He says his opinion -- reflected in current federal guidelines -- is based on scientific evidence from hundreds of studies.
Now debate is growing about that evidence, and about who should be entrusted to interpret it. Some public-health officials say industry-funded doctors are ignoring other studies that suggest cold turkey is just as effective or even superior to nicotine patches and other pharmaceuticals over the long run, not to mention cheaper.
At stake is one of the most important issues in the nation's public-health policy. Cigarettes kill an estimated 440,000 Americans a year. Helping America's 45 million smokers kick the addiction could save untold numbers of people.
The Public Health Service, part of the Department of Health and Human Services, issued guidelines in 2000 calling for smokers to use nicotine patches, gums and other pharmaceutical aids to quit, with a few exceptions such as pregnant women. Dr. Fiore, a University of Wisconsin professor of medicine, headed the 18-member panel that created those guidelines. He and at least eight others on it had ties to the makers of stop-smoking products.
Those opposed to urging medication on most quitters note that cold turkey is the method used by the vast majority of former smokers. They fear the federal government's campaign could discourage potential quitters who don't want to spend money on quitting aids or don't like the idea of treating their nicotine addiction with more nicotine.
"To imply that medications are the only way is inappropriate," says Lois Biener, a senior research fellow at the University of Massachusetts at Boston who has surveyed former smokers in her state. "Most people don't want them. Most of the people who do quit successfully do so without them."
Guidelines Revision
The panel is now working on a revision of the guidelines, scheduled for completion early next year. Dr. Fiore, an internist, is again chairman. He says this time only seven of 26 members have industry ties. Karen Migdail, a spokeswoman for the revision effort, says it involves so many voices that "it's hard for one perspective to have an influence on the process." She says Dr. Fiore is "one of the leading experts" in smoking cessation and well-suited to the job.
RELATED READING
|
Dr. Fiore says his panel will give a fair hearing to all points of view on smoking cessation. He says the process is sufficiently collaborative to prevent bias, his or anyone else's, from creeping into the final product. He notes that many of the studies questioning the effectiveness of stop-smoking medication arose after the publication of the 2000 guidelines. The panel will scrutinize them closely before reaching any conclusions, he says.
David Blumenthal, director of the Institute for Health Policy at Massachusetts General Hospital, questions the government's choice of Dr. Fiore. "The chairman of the committee should be unquestionably impartial," says Dr. Blumenthal, who has published extensively on conflicts of interest.
Pharmaceutical companies make several products to help smokers quit. Some give a nicotine fix without a cigarette, such as GlaxoSmithKline PLC's Nicorette gum and nicotine-laced Commit lozenges. Nicotine, the addictive agent in cigarettes, is considered benign relative to the carcinogens in cigarettes. Bupropion, an antidepressant, and Pfizer Inc.'s Chantix -- both pills available only by prescription -- aim to reduce cravings without using nicotine.
Many clinical trials have randomly assigned smokers to take one of these products or a placebo. Such randomized trials are considered the gold standard in many medical fields, and they have consistently shown that nicotine-replacement therapy or other medicine confers a benefit.
But these trials have limitations. They tend to compare quitters who wanted medication and got it with those who wanted medication and didn't get it -- which is a different group from quitters ready to try going cold turkey. Also, clinical trials tend to attract highly motivated quitters who may not represent the population as a whole. Even the placebo group in these trials often boasts double the success rate of the population of quitters generally.
Studies of quitters outside clinical trials have shown no consistent advantage for medicine over cold turkey, the pharmaceutical industry's primary competitor. An unpublished National Cancer Institute survey of 8,200 people who tried quitting found that at three months, users of the nicotine patch and users of bupropion remained abstinent at higher rates than did users of no medication. But at nine months, the no-medication group held an advantage over every category of stop-smoking medicine. The study was presented at a world tobacco conference last summer.
Real-World Situations
Similar so-called population studies -- which review results of people who already quit or tried to, rather than prospectively randomizing subjects into groups -- have also suggested that cold-turkey quitting can compete with medication in real-world situations. These studies, in California, Massachusetts and Australia, have their own limitations. One is that they depend on people to remember what they did rather than monitoring them in a controlled experiment.

|
| The surgeon general's five-day program for smokers preparing to quit recommends nicotine patches or other medication. |
Kenneth Strahs, GlaxoSmithKline's vice president of smoking-control research and development, notes that his company's products won approval from regulators at the Food and Drug Administration who demand randomized clinical trials. "The FDA does not conclude either safety or efficacy based on retrospective population studies," says Dr. Strahs. Smoking-control products account for a small fraction of the company's revenue.
The researcher who raised the first serious questions about nicotine-replacement therapy says it may fall into a rarely discussed gap between efficacy in clinical trials and effectiveness in the real world. Greater use of medication is not "associated with any increase in successful quitting in the population," says John Pierce, a University of California, San Diego, professor of medicine who was lead author of a 2002 Journal of the American Medical Association article finding no superior benefit from over-the-counter nicotine substitutes in California.
"If we're going to be intellectually honest, we have to be willing to examine the issue of whether current users [of medication] are obtaining long-term rates of abstinence that are higher than anyone else," says Kenneth Warner, a tobacco researcher and dean of the University of Michigan School of Public Health. "That's going to be very hard for people to do in the smoking-cessation community," because belief in the value of medication runs so deep, he adds.
All sides in the debate agree that intervention by doctors and other health-care providers to confront smokers can be effective in encouraging quitting. Dr. Fiore says the primary goal of the guidelines is to spur such intervention, and he says they have been successful in sharply raising the proportion of doctors who discuss smoking with their patients. Also undisputed is that behavioral support, whether from professional therapists or quit-line counselors, can be valuable.
As the federal government weighs the data in making new recommendations, many of its advisers are receiving money from companies with a stake in the outcome. Dr. Fiore holds a chair at Wisconsin that is funded by GlaxoSmithKline. He directs a tobacco research center that received nearly $1 million in funding from makers of quit-smoking medicine in 2004 and $400,000 in 2005. Between 1999 and 2004, Dr. Fiore personally pocketed $10,000 to $40,000 a year from the quitting-aid industry for honorariums and consulting work. He says he stopped such work in 2005.
In the U.S. government's 2005 civil case against the tobacco industry, it chose Dr. Fiore as an expert witness. He was asked to estimate the damages owed to federal taxpayers as a result of smoking and to devise a plan for spending those damages. Dr. Fiore came up with an estimate of $130 billion, and a plan to spend about $5.2 billion a year of that mostly on counseling and medication -- a measure that could have doubled the size of the stop-smoking medicine market. (Later, the government reduced its request for damages to $10 billion.)
The American Cancer Society has allowed its logo to be placed on stop-smoking products in exchange for money. A Cancer Society spokesman defends that decision, crediting the pharmaceutical industry for bringing invaluable marketing muscle to the society's Great American Smokeout every November.
Those who advocate medication sometimes fail to disclose that they have financial ties to companies. In an article on Voice of America's Web site last year, Jack Henningfield, identified only as a smoking-cessation expert, urged smokers to "go to the consumer-friendly Web site that I like, which is www.quit.com."
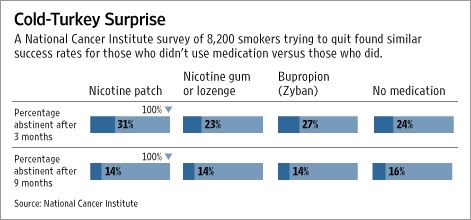
Dr. Henningfield is a principal of Pinney Associates, a consulting firm whose largest client is GlaxoSmithKline, operator of the quit.com site. Other articles citing Dr. Henningfield's views on smoking have identified him as a professor at Johns Hopkins School of Medicine without mentioning the GlaxoSmithKline connection. Dr. Henningfield, who holds a doctorate in psychology, is an adjunct professor at Johns Hopkins. He says only 10% of his income comes from Hopkins.
Dr. Henningfield says he always tells journalists about his financial ties to the industry. But in an interview with The Wall Street Journal last summer, Dr. Henningfield promoted the use of stop-smoking medicine without volunteering any information about those ties. He says he thought GlaxoSmithKline's public-relations firm had already provided the information.
In at least two medical-journal articles that Dr. Fiore wrote or co-wrote promoting the use of stop-smoking medicine, no mention was made of his financial ties to the makers of those treatments. Dr. Fiore says the editors of those journals may have ignored his disclosure or he may have failed to provide it. If the latter, "I am sorry about that," he says, adding that those are two of more than 150 medical-journal articles he has published.
Dr. Fiore and other members of the Society for Research on Nicotine and Tobacco refuse to accept any funds from the tobacco industry, even unrestricted research grants. Smoking-control activists say there's a big difference between tobacco companies, which they say engaged in scientific deceit for a half-century, and drug makers that are trying to help smokers quit. Reflecting the view of many in the antitobacco camp, Harry Lando, a University of Minnesota nicotine researcher, says, "I view the pharmaceutical industry as our ally."
After the federal panel with industry-funded scientists came out with its guidelines in 2000, a campaign against cold turkey took root. The Web site of the highest-ranking physician in America -- the surgeon general -- calls it a "myth" that cold turkey is the best way to quit. In November 2006, during the week of the Great American Smokeout, doctors around the country participated in a campaign called "Don't Go Cold Turkey." The creator of the campaign was GlaxoSmithKline.
Advocate Rejected
The how-to-quit Web site of the federal Centers for Disease Control and Prevention rejected a request from John Polito, an ex-smoker in Mount Pleasant, S.C., to include a link to his Web site, WhyQuit.com, which advocates cold-turkey quitting. In a 2002 letter explaining the rejection, the agency told Mr. Polito that drug therapy has been shown to double quit rates.
In an interview, CDC epidemiologist Corinne Husten said the real reason for the rejection is that the CDC doesn't recommend private Web sites. However, the CDC site long included a link to GlaxoSmithKline's quit.com site. Asked about that, Dr. Husten said, "Some things have gotten on the [CDC] Web site that shouldn't be there." (After the interview, the CDC removed the quit.com link.)
Pressure may be growing for doctors to follow the federal guidelines. An article in the December issue of the journal Tobacco Control argued that failure to follow the guidelines could be deemed medical malpractice.
Some health officials don't go along with the federal government's tilt against cold turkey. The state of California's help-line for smokers presents cold turkey as an equally viable option to medication. "The effectiveness of pharmaceutical aids has been proven short-term; long-term, it's still in debate," says Hao Tang, a research scientist with the state department of health services. California has succeeded in reducing its smoking rate to 14%, six percentage points below the national average.
After three decades of smoking, Linda Holstein quit nearly three years ago using a nicotine patch as well as nicotine gum, which on occasion she still pops into her mouth. Elated at being free from cigarettes, Ms. Holstein, a Minneapolis attorney, says, "The gum helped very much."
Others say ingesting medicinal nicotine prolonged withdrawal, leading them ultimately back to cigarettes. During the 20 years that Tanya Blakey, a Georgia teacher, smoked two packs a day, she tried to quit countless times using nicotine-replacement therapy. "Every time I stopped using the NRT, I was smoking again within two or three days," says Ms. Blakey. This week she is celebrating two years without a cigarette, this time having used no medication.
Write to Kevin Helliker at kevin.helliker@wsj.com
Related Reading
- Impact of Over-the-Counter Sales on Effectiveness of Pharmaceutical Aids for Smoking Cessation, JAMA. 2002;288:1260-1264.
- Nicotine replacement therapy for smoking cessation - The Cochrane Database of Systematic Reviews, 2006 Issue 4
- What Does U.S. National Population Survey Data Reveal About Effectiveness of Nicotine Replacement Therapy on Smoking Cessation? - NCI, US DHHS (WCTOH 2006 PPT)
- You can quit smoking - US Surgeon General (May 2003)
Is the U.S. Public Health Service Listening?
 The CDC reports that for the first time since 1997 the U.S. smoking rate has stalled and failed to decline. The lives of 45.1 million Americans are at risk. While openly admitting that nearly 90% of all long-term successful quitters quit smoking cold turkey, their government continues to pound, pound into their brains the message that their natural instincts -- to stop using all nicotine -- are wrong, that they should not stop using nicotine but replace it. Their confidence has been shattered and lives are being lost.
The CDC reports that for the first time since 1997 the U.S. smoking rate has stalled and failed to decline. The lives of 45.1 million Americans are at risk. While openly admitting that nearly 90% of all long-term successful quitters quit smoking cold turkey, their government continues to pound, pound into their brains the message that their natural instincts -- to stop using all nicotine -- are wrong, that they should not stop using nicotine but replace it. Their confidence has been shattered and lives are being lost.
Insane as it may sound, this is our nation's all consuming cessation policy as reflected in June 2000 Guideline Recommendation 7, a policy written by a panel on which 11 of 17 members openly acknowledged financial ties to the pharmaceutical industry. And this is 2008 Guideline Update Recommendation 6, written by many of the same financially conflicted pharma consulants.
This critically important article by Kevin Helliker is the first to expose the fact that while quitting pharmacology products reign supreme inside highly manipulated double-blind randomized clinical trails, they fall flat on their face in real-world use, failing to perform as well as those quitting without them. Sadly, the U.S. Public Health Service is deeply involved in serious and life-threatening deception of smokers. But why?
Experienced nicotine addicts know when their nicotine delivery device is an empty placebo. They can sense when their withdrawal syndrome begins and whether it is easier or as hard as the last time. Even though a "few" NRT studies assert otherwise (very few), common sense screams that it is impossible to blind clinical studies involving chemical withdrawal from highly addictive drugs but that hasn't stopped the pharmaceutical industry from making billions in profits by pretending success at doing so. If you were a nicotine addict expecting relief, would you have stayed and allowed yourself to be toyed with? Neither did they. Imagine hundreds of millions spent on NRT studies, and nearly all totally meaningless.
Is our government knowingly engaged in defrauding smokers out of priceless periods of cessation confidence? Fraud requires that they "knew or should have known" that deceit was being committed. It clearly knows that in no NRT study have NRT users ever gone head to head with a quality brupt nicotine cessation education or support program. The outcome would be sadly laughable - extremely sad. So instead, as Kevin Helliker points out, our government assists in bashing, trashing, ignoring or even hiding the art, science and psychology underlying successful abrupt nicotine cessation. But why?
We can only hope that the Public Health Service is taking Mr. Helliker's piece seriously but don't hold your breath. As noted in the story, at this moment a number of panel members with financial ties to quitting products are engaged in revising the U.S. smoking cessation Guideline. It is against their financial interests to find that real-world effectiveness is probably more important than clinical efficacy inside double-bind clinical trials that were not blind. Read pages 14 and 15 of Fiore's May 2005 testimony in U.S. vs. Philip Morris, USA and then ask yourself this, if Fiore loses favor with the cessation pharmaceutical industry how much does he stand to lose?
The Agency for Healthcare Research and Quality (AHRQ) is the U.S. Public Health Service gatekeeper tasked with determining what is and isn't effective. The AHRQ is the Agency allowing those with financial ties to pharmaceutical interests to revise the Guideline. If U.S. federal law bars federal employees from participating personally and substantially as a government employee in any matter in which the employee has a financial interest (see 19 U.S.C. 208), how can they appoint a person with declared financial interests to do what they themselves cannot? The penalty for wilfull violation of the financial interest statute is up to five years imprisonment and/or a civil penalty of up to $50,000 for each violation. The Director of AHRQ is Carolyn M. Clancy, M.D. (biography) and her e-mail address is Carolyn.Clancy@ahrq.hhs.gov. If this story angers you please send Dr. Clancy a quick email and let her know. She works for us and she also took an oath to "never do harm to anyone."
John R. Polito
Editor WhyQuit.com
Related WhyQuit Articles
- Is the U.S. government's quitting policy killing smokers?
- The secret to quitting smoking
- 90% of ex-smokers quit smoking cold turkey
- Chantix - an 8 in 10 failure rate or worse?
- Will Chantix really help me quit smoking?
- 13th World Conference on Tobacco or Health Drenched in Nicotine
- Cold Turkey Twice as Effective as NRT or Zyban
- Nicotine Not Medicine, Its Use Not Therapy
- GlaxoSmithKline Attacks Cold Turkey Quitting
- The Nicotine Patch, Gum and Lozenge - Mounting Evidence of a Sham
- Widespread Blinding Failures Put NRT Studies in Serious Question
- Nicotine Gum Maker's Concern Raises Concerns
- Quebec CT Quitters Disprove "Double Your Chances" NRT Assertion
- Are nicotine weaning products a bad joke?
- Is CT Quitting More Productive & Effective Than NRT?
- Are Teens Getting Hooked on NRT?
- March 2003 OTC NRT Meta-Analysis Finds 93% Midyear Relapse Rate
- JAMA Study Concludes NRT is Ineffective
- Real-World Nicotine Patch and Gum Rates
- Does the OTC Nicotine Patch Really Double Your Chances of Quitting?
- Is Nicotine Replacement Therapy The Smoker's Last Best Hope?
Knowledge is a Quitting Method
