Studies screaming "leave replacement nicotine alone!"
Editor's Note: Originally this article featured 10 studies but has updated periodically adding new findings.
Updated 12/31/22 by John R. Polito
 How is a nicotine addict attempting to wean themselves off of nicotine any different than the alcoholic toying with alcohol weaning?
How is a nicotine addict attempting to wean themselves off of nicotine any different than the alcoholic toying with alcohol weaning?
Imagine an alcohol replacement therapy clinical trial. Imagine the accomplishment of alcoholics who successfully transferred their dependency from a bottle or beer can to an IV drip bag being compared to alcoholics who had abruptly ended alcohol use weeks or months earlier, when given IV bags containing sugar water (the placebo group). How long would it be before someone shouted fraud?
Why do nicotine replacement therapy products (NRT) - the nicotine patch, gum and lozenge - prevail inside randomized clinical trials yet fail miserably in real-world use?
The loser within clinical trials was a placebo group using look-a-like NRT products. What the pharmaceutical industry would rather you not know is that the placebo gum (1982) or placebo patch (1996, 1997, 2002) was often spiked with just enough nicotine to keep users in the tease and throws of withdrawal: not delivering enough to satisfy cravings, nor allowing them to get clean, begin re-sensitizing, and move beyond peak withdrawal.
Studies not using active placebos were not blind as claimed. It's why active placebos were needed. Researchers knew that if participants were aware of their assignment the outcome would reflect expectations, not product worth.
Smokers with lengthy quitting histories become experts at recognizing the onset of withdrawal. The few quality blinding integrity assessments shared in clinical studies show that three (1997, 2005) to four (2009) times as many placebo group members could correctly declare their randomized assignment as declared wrong, and could do so within 24-48 hours of quitting (peak withdrawal).
Would you have become frustrated if you'd joined a study hoping to receive weeks or months of free NRT, only to realize that you'd been given a placebo instead? So did many of them.
Aside from frustration handing NRT victory by default, counseling, support and other study contacts were designed to at first foster successful dependency transfer from smoking to NRT, and then to gradually wean NRT users off of nicotine. What the inactive placebo group needed and was deprived of was counseling, guidance, and support tailored to the needs of someone abruptly ending nicotine use.
Real-world NRT quitters are not trained, counseled, and supported by NRT experts. But then, neither are real-world cold turkey quitters. Worldwide, relatively few cold turkey quitters study at cold turkey quitting schools such as WhyQuit.
That's what makes the below real-world studies critical, the fact that unassisted cold turkey quitters generate substantially higher long-term success rates than unassisted NRT quitters.
What NRT stakeholders can never do is conduct fair studies that pit quitters educated and schooled in successful abrupt nicotine cessation against quitters educated and schooled in nicotine dependency transfer and weaning. Why? Because, as shown below, even with delayed nicotine cessation, NRT gets clobbered in "almost" fair fights.
Truth is, all long-term independent real-world population level quitting method studies since 2002 have found that NRT simply doesn't work. As I suggested in a 2012 British Medical Journal letter, NRT has undercut successful quitting and is costing lives.
 So, how does pharma get away with selling products that don't work? It relies upon an army of paid academic consultants (what Professor Chapman calls the "PhD industry") to help hide, suppress or dismiss as non-science-based any and all evidence of NRT ineffectiveness. It then helps market NRT by knowingly pretending the fiction that efficacy and effectiveness are one and the same.
So, how does pharma get away with selling products that don't work? It relies upon an army of paid academic consultants (what Professor Chapman calls the "PhD industry") to help hide, suppress or dismiss as non-science-based any and all evidence of NRT ineffectiveness. It then helps market NRT by knowingly pretending the fiction that efficacy and effectiveness are one and the same.
NRT marketing often includes the assertion that NRT doubles "your" chances. The word "your" clearly suggests population effectiveness, not efficacy victories over placebo.
Common sense shouts that smokers wishing to quit cold turkey do not join NRT studies dangling free "medication" or NRT as study recruiting bait. Nor do they use placebo NRT products laced with small amounts of nicotine. The only time NRT has gone head-to-head with cold turkey is out in the street.
And what happens there? Nicotine's half-life inside the body is two hours. It means that within 72 hours of ending all use, the cold turkey quitter becomes 100 percent nicotine-free. Their brain receptors will begin re-sensitizing and they'll have no choice but to move beyond peak withdrawal within the first 3 days.
The pharmaceutical industry's economic muscle and influence is massive, as evidenced by the millions it annually hands to researchers, consultants, universities, medical journals, and to both government and public health organizations.
Could that muscle explain why a former Nicorette salesman was chosen to head the Food and Drug Administration's Center for Tobacco Products, or why the nation's telephone quitline nicotine patch give-a-way champion was appointed director of the Centers for Disease Control's Office on Smoking and Health?
Could it also be why quitters will not find reference to any of the following population-level studies on any website advocating NRT use or in any nation's guideline reviewing evidence and establishing smoking cessation policy? Could it be that the AHRQ, FDA, CDC, NCI, Surgeon General, NHS and NICE are all on the wrong side (pharma's) in the war against smoking?
Key Studies Warnings Against NRT Use
- December 2022 - Preventive Medicine Reports: Among 2,783 French quitters, the overwhelming majority, 61.7% (1,716) attempted to quit unassisted, without the use of e-cigarettes, NRT or any other quit smoking product. According to Table 2, unassisted quitters were 27% more likely than NRT quitters to have successfully quit smoking for 6 months, were 38% more likely to succeed for a year, and 43% more likely to quit for 2 years.
- July 2022 - Addictive Behaviors: Among 1,045 adult smokers in England who provided data at baseline (April 2015-November 2020) and reported a serious past-year quit attempt at 12-month follow-up, the study's raw data indicates that quitting without use of any quitting aid was 43 percent more likely to succeed than use of over-the-counter NRT (odds ratio, 0.57, 95% CI, 0.39–0.84) and 40 percent more likely than prescription NRT (odds ratio, 0.60; 95% CI, 0.37–0.98).
- March 2022 - Current Oncology: Among 186 mostly heavy long-term smoking patients diagnosed with head or neck cancer, at six months 13 patients had died and 41 had quit smoking. Most, 51.2%, successfully quit cold turkey with 14% quitting via NRT.
- July 2021 - Urology: Among 151 active smokers diagnosed with bladder cancer, 115 made a quitting attempt with 65 or 56% being successful. Among the 65 successful quitters, 42 or two-thirds (66%) quit cold turkey
- September 2020 - PLoS One: A cohort wave study involving 2,443 US adult smokers who participated in the PATH survey and reported a quit attempt at annual follow-up. According to study Table 1, smokers quitting without the use of any pharmaceutical aid were 24% more likely to be abstinent from all forms of tobacco after 12 months, including e-cigs, than those using FDA-approved quitting products. As for ex-smoker productivity, unassisted quitting produced 4.2 times as many success stories as approved products. Link to free full-text.
- May 2019 - Addiction: Comparing 8,348 unassisted quitters to 5,206 OTC NRT quitters, this England Smoking Toolkit Study (STS) found an unadjusted smoking abstinence rate of 16.8% for unassisted quitters and 11.6% for OTC NRT quitters. Link to free full-text.
-
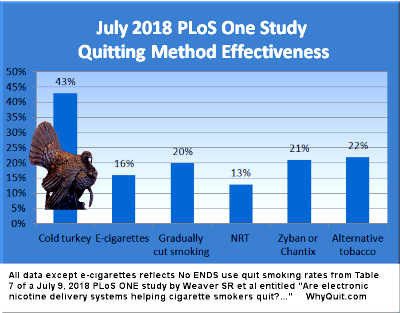
July 2018 - PLoS One (free full-text): Taken at face value, Table 7 of this prospective population-based study indicates that quitting smoking cold turkey was not only 11 times more productive than nicotine replacement therapy (NRT) in producing successful quitters (56 cold turkey quitters vs. 5 NRT quitters), it was also 3.3 times more effective (43.4% vs. 13.1%). Unfortunately, as my official comments regarding the study indicate, the study's definition of "cold turkey" was abrupt smoking cessation, not abrupt nicotine cessation. Thus, quitting method crossover would likely change the above figures somewhat but probably not much.
- December 2017 - Journal of the National Cancer Institute: A propensity matched effectiveness study using 12 potential confounders (age, sex, race-ethnicity, education, smoking intensity, nicotine dependence, previous quit history, self-efficacy to quit, smoke-free homes, survey year, and cessation aid use) of 2,219 pharmaceutical aid users and nonusers participating in 2002-2003 and 2010-2011 TUS-CPS surveys found that "there was no evidence that use of varenicline, bupropion or nicotine replacement increased the probability of 30 days or more smoking abstinence at one-year follow-up."
- October 2014 - Mayo Clinic Proceedings: A prospective population-level study involving the UK's previously most driven and dedicated NRT advocate (Robert West, PhD) found that "Compared with smokers using none of the cessation aids" at 6-month follow-up" ... "use of NRT bought over the counter was associated with a lower odds of abstinence (odds ratio, 0.68; 95% CI, 0.49-0.94)."
- July 2013 - Gallup Poll: After 3 decades of heavy nicotine gum marketing, this national survey found that only 1 in 100 successful ex-smokers credit nicotine gum for their success, that only a tiny fraction quit by use of any approved product (just 8%), and that more quit smoking cold turkey than by all other methods combined.
- January 2012 - Annual Review of Public Health: This study reviewed U.S. government survey data and found that, whether a light smoker (less than 15 cigarettes per day) or a heavy smoker (greater than 15 per day), that replacement nicotine is ineffective when compared to smokers quitting unassisted.
- July 2009 - Nicotine & Tobacco Research: By definition, the purchase and use of nicotine patches, gum or lozenges requires planning. This study involved one of the leading U.S. NRT advocates, Saul Shiffman, PhD. Nearly identical results to an earlier 2006 UK study, it found that unplanned quit smoking attempts were 2.6 times more likely to succeed for 6 months than planned attempts. It's why any website asserting that planning is "key" to success is a quitting product storefront (a claim being made by both Philip Morris USA and the CDC).
- May 2006 - Addictive Behaviors: Patient smoking and quitting data of 1,000 Australian family practice physicians was gathered and analyzed. Not only was cold turkey quitting by far the most effective method - doubling the success rate of nicotine gum, nicotine patch and nicotine inhaler quitters - it was by far the most productive method. Successful cold turkey quitters accounted for 1,942 of 2,207 former smokers, a whopping 88% of all success stories.
- 2006 - National Cancer Institute - A February 8, 2007 front-page Wall Street Journal article by a Pulitzer Prize winning author featured a study by the National Cancer Institute. The Institute's study examined 8,200 quitters and found that at 9 months those quitting without use of approved products had a slightly higher rate of success than those using the nicotine gum, patch, lozenge or Zyban. Unpublished and not available on any government website, the study evidences actual government awareness and cover-up of NRT ineffectiveness.
- 2005 - Journal of Addictive Disorders: Among 1,954 questionnaire respondents, "when NRT use was assessed in relation to smoking status in 1998, 30% of NRT ever users compared to 39% of nonusers had quit smoking."
- June 2004 - Addictive Behaviors: This study reviewed NRT blinding integrity assessments. It should have alerted both the FDA and CDC to the fact that NRT studies were not blind as claimed, in that 12 of 17 studies "found that subjects accurately judged treatment assignment at a rate significantly above chance. U.S. health officials should have immediately demanded that all future studies funded by taxpayers include a blinding integrity assessment."
- March 2003 - Tobacco Control: A study by GlaxoSmithKline paid consultants combined and averaged all U.S. over-the-counter nicotine patch and gum studies. It found that 93 percent of OTC NRT quitters had relapsed to smoking within 6 months.
- 2003 - Cancer Facts & Figures 2003: Table 3 on page 25 of this American Cancer Society report shares data findings from the 2000 national surveys. It indicates that 91.2% of former U.S. smokers quit smoking cold turkey. This was 16 years after nicotine gum became a cornerstone of U.S. cessation policy.
- September 2002 - Journal of the American Medical Association: This study involved a large population level quit smoking survey sampling. It concluded that "Since becoming available over the counter, NRT appears no longer effective in increasing long-term successful cessation in California smokers."