
Replacement nicotine's killing fields
John R. Polito
 The vast majority of smokers remain unaware of cold turkey's continuing rein as real-world quitting champ. Still, for smoking cessation policymakers, counselors and researchers, today marks a new page in the quitting method debate. For the first time, a major medical journal has dared pose the until now only whispered question: is toying with pharmaceutical grade replacement nicotine (NRT) costing smokers their lives?
The vast majority of smokers remain unaware of cold turkey's continuing rein as real-world quitting champ. Still, for smoking cessation policymakers, counselors and researchers, today marks a new page in the quitting method debate. For the first time, a major medical journal has dared pose the until now only whispered question: is toying with pharmaceutical grade replacement nicotine (NRT) costing smokers their lives?
Today's February 11, 2012 print edition of the British Medical Journal (BMJ) contains a letter entitled "Are those who quit smoking paying with their lives because of NRT's failure?"1 The letter's author shares four population level NRT defeats yet fails to answer the question posed. Limited by the BMJ to 250 words, as the letter's author I do so here.
NRT simply doesn't work. Brain dopamine pathways cannot adjust to functioning without nicotine while it continues to arrive. Users don't quit because of NRT, but in spite of it. And, yes, replacement nicotine is both directly and indirectly costing lives.
Replacement Nicotine's Real-World Failure
While NRT generally clobbers users of placebo NRT look-a-likes inside randomized clinical trials, placebo isn't a real quitting method. In long-term studies by non-conflicted independent researchers since 2000, once outside clinical trial doors NRT has consistently failed to prevail over four categories of quitters: (1) cold turkey quitters,2 (2) unassisted quitters,3 (3) non-medication quitters,4 and non-NRT quitters.5
Each of these population level comparison categories generates different outcomes. Roughly twice as effective, cold turkey hands NRT its greatest defeat (see Doran 2006). Unassisted lumps abrupt cessation (cold turkey) with less effective gradual weaning/tapering. Non-medication lumps cold turkey with hypnosis, magic herbs and acupuncture. Non-NRT lumps cold turkey with every non-NRT quitting method, including Zyban, Chantix/Champix, Smoke Away and E-Z Quit.
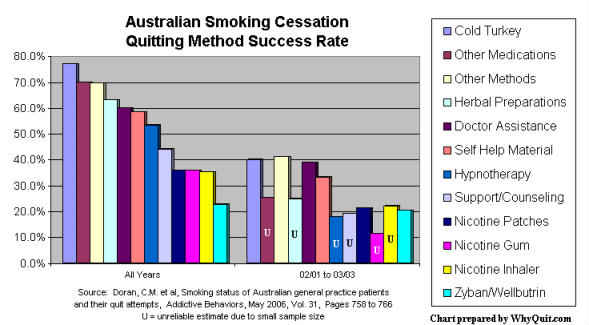
Although almost always masterfully spun, even research by paid and highly conflicted pharmaceutical industry consultants can no longer hide the fact that smokers continue to be lied to about how most quitters succeed, and "their" real-world chances with and without NRT.6
But these glorified NRT salesmen are fighting back. Imagine the real-world data mud created by drafting and asking a quitting method survey question in which none of the 20 listed quitting options is cold turkey, the planet's most popular and productive method.7 Imagine forcing a proud cold turkey quitter to listen to 18 other quitting method options before compelling them to select either option 19 or 20, "nothing" or "other."
Today, an army of conflicted researchers is doing its best to get policymakers to ignore NRT's worthlessness. They have two current favored arguments: (1) that population study findings are unworthy of belief because unassisted quit smoking attempts are easily forgotten, while attempts where NRT, Zyban or Chantix is purchased and used are difficult to forget; and (2) that heavily dependent smokers tend to gravitate to use of approved quitting products thus making their failure rates higher, also known as selection bias.
Both the "better recall" and "stronger addiction" arguments were made Tuesday in the industry's most favored journal, Nicotine and Tobacco Research. There, it was concluded that both factors could bias surveys.8
The forgotten attempts argument is a misdirection red herring which focuses our attention upon failure instead of success. While entirely true that failed cold turkey attempts are easily forgotten, try to locate any successful cold turkey quitter who cannot recall how they were at last able to succeed. Good luck in finding one.
The "more strongly addicted" or "selection bias theory" is another red herring. First, the 2008 Guideline Update reflects official government quit smoking policy. It openly and repeatedly declares that "cessation medications have not been shown to be beneficial to light smokers" smoking a half a pack per day or less.9 If the only beneficiaries of NRT are more strongly addicted smokers, how can the industry claim bias and foul when NRT fails its intended population?
More importantly, last month a new population level study put a rather big dent in the selection bias theory. It found that among heavily dependent quitters, that those using NRT had 3.5 times greater odds of relapse than those quitting without it. The study defined "heavily dependent" as having smoked within 30 minutes of waking + smoking at least 20 cigarettes per day.10
Direct NRT Deaths
 The average smoker only has so many priceless periods of cessation confidence before paying smoking's ultimate price. If
Doran 2006 is correct and cold turkey is substantially more effective than NRT, quitting attempts squandered on replacement nicotine have likely contributed to hundreds of thousands of premature smoker deaths.
The average smoker only has so many priceless periods of cessation confidence before paying smoking's ultimate price. If
Doran 2006 is correct and cold turkey is substantially more effective than NRT, quitting attempts squandered on replacement nicotine have likely contributed to hundreds of thousands of premature smoker deaths.
According to the Centers for Disease Control (CDC), the United States has 45.3 million adult smokers.11 During 2010, 52.4 percent of current adult smokers made a quitting attempt of at least 24 hours. We also know that 30 percent who tried quitting in the past year used NRT, Zyban or Chantix.12
Thus, we know that during 2010 approximately 23.7 million Americans made a serious quitting attempt of at least 24 hours and that approximately 7 million used NRT, Zyban or Chantix. And what about Chantix? Although Chantix has repeatedly proven superior to Zyban (bupropion), is Chantix more effective than NRT under real-world conditions? Early evidence suggests not.
There have only been two randomized clinical trials pitting Chantix against NRT, Aubin 200813 and Tsukahara 2010.14 In both, Chantix failed to show statistical significance over nicotine patch quitters when assessing the percentage of users within each group who were not smoking at 24 weeks.
Let's assume for the sake of discussion purposes only that 2010's roughly 7 million NRT, Zyban and Chantix quitters had a real-world one-year quitting rate of 6 percent, versus 9 percent if they would have quit cold turkey. These assumptions would produce 420,000 successful NRT, Zyban and Chantix quitters versus 630,000 if they'd quit cold turkey.
Thus, in one year alone, 210,000 failed NRT quitters would have needlessly continued smoking until their next period of quitting confidence, if any.
Last year our online quit smoking site lost two successful cold turkey quitters to lung cancer, Neil age 53 and Helen age 50.15 Prior to being diagnosed with cancer, both were angry about the years of freedom that replacement nicotine had robbed from them.
Neal - "My past quit attempts left me addicted to other nicotine delivery devices," wrote Neil prior to his diagnosis. "Leading up to this quit I had been addicted to nicotine lozenges for over a year, I used them and smoked cigarettes constantly. When I couldn't have a cigarette I would have a lozenge in my mouth. Occasionally I would smoke cigars. I tried the e-cigarettes, the inhalers, the gum, the patch, and I even tried oral tobacco to break up the routine. It was a real nicotine addict's heaven, but in reality it was hell."
On April 9, 2011, Neal wrote that the "real me still wanted so badly to surface and be free from the deep, dark, jungle. I tried and tried and even enlisted the help of gums, lozenges, patches, inhalers, e-cigs, and dip. I found myself in worse shape then when I started. Not only was I still buried in the jungle, but now the lozenges were growing vines around my legs, and I could barely move."
"I originally named my first post journal '4 Decades Of Lies' because I have been smoking for over 40 years," wrote Neil on February 9, 2011. "The lies I was talking about was aimed at the tobacco and pharmaceutical companies, and the government for letting them do all that lying to us."
Helen - "I want to ask why is it that we think that NRT works," wrote Helen prior to her diagnosis. "Because pharmaceutical companies tell us it works? And who stands to gain from that?"
"I used NRT twice, the first time was maybe about fifteen years ago. My friends and family were all on my case to quit. I used the patches for the full three months. Then, when it was time to stop using the patches, I started smoking again," recalled Helen.
"The second time I used NRT was when my sister was diagnosed with breast cancer. She really got on my case. She was constantly reminding me, 'Helen, smoking causes cancer!' This time I wasn't able to stop smoking even while wearing the patch. Each week the quitting nurse I was working with would tell me to not smoke while wearing the patch, but I just couldn't stop. In the end we just gave up on each other."
"When the doctors, nurses and media kept telling me that the patch doubles my chance of quitting I believed them," wrote Helen. "These guys are the professionals so they must be right. I'm the dropout so it must be all my fault that I can't stop smoking. So instead of jumping up and down and complaining that their product is faulty, I went away resigned to having to smoke for the rest of my life."
Indirect NRT Induced Deaths
And therein lies NRT's deadly rub, that Neil, Helen and millions of other NRT quitters failed after having tried the very best that science had to offer. They believed NRT's double your chances lies and walked away not only defeated but feeling hopeless.
In fact, it is extremely difficult to find any government quitting literature that doesn't cram the double "your" chances lie down the throats of quitters.
Imagine being a cold turkey quitter and visiting SmokeFree.gov, the U.S. government's official quit smoking site, where 173 times you are bombarded with the message to use "medication" or "medicine."16
Imagine constantly being assaulted with the false suggestion that your natural quitting instincts are all wrong, that cold turkey is ineffective, that few succeed. How many cold turkey quitters became demoralized and threw in the towel because recent NRT commercials daily hammered home the message that "quitting sucks" and that NRT makes it suck less?
Today, despite cold turkey generating more successful ex-smokers than all other quitting methods combined, there is no government quit smoking site or handout offering cold turkey quitters encouragement or support. None.
 The U.S. government's primary quit smoking booklet is"Clearing the Air." The "Cold Turkey" section of Clearing the Air states, "For some smokers, 'going cold turkey' seems like the easiest way to quit: Just stop smoking and tell yourself you'll never light up again. This works for some smokers - usually those with the lowest level of nicotine dependence - but not many. Fewer than 5 percent of smokers can quit this way."
The U.S. government's primary quit smoking booklet is"Clearing the Air." The "Cold Turkey" section of Clearing the Air states, "For some smokers, 'going cold turkey' seems like the easiest way to quit: Just stop smoking and tell yourself you'll never light up again. This works for some smokers - usually those with the lowest level of nicotine dependence - but not many. Fewer than 5 percent of smokers can quit this way."
Again, imagine being a cold turkey quitter and reading those words. Ask yourself, is it fair to assert that this booklet suggests that only 1 in 20 smokers have the ability to quit cold turkey? Why would U.S. government health officials lie to quitters? What possible motivation could health officals have for attempting to motivate and induce cold turkey quitters to relapse?
I submit that industry and government cold turkey bashing, and government neglect of cold turkey quitter counseling and support needs, helps fuel and motivate countless thousands of annual cold turkey relapses, resulting in the indirect killing of smokers.
But there's another and possibly larger indirect NRT killing field. It's replacement nicotine's interruption of natural learning of the most important stop smoking lesson of all, that lapse nearly always equals relapse, that one equals all, that one puff is too many, while thousands never enough.
Natural relapse learning eventually teaches us nicotine addicts the same relapse prevention lesson eventually gleaned by the alcoholic, that there's no such thing as just one sip or puff.
The pharmaceutical industry muddies natural learning with the contrary lesson that nicotine is medicine and its use therapy. Reflect on the insanity of teaching alcoholics that alcohol is medicine and its use therapy.
In that NRT actually undercuts natural cessation rates, mere mention of "nicotine" and "therapy" in the same breath is itself extremely destructive of natural relapse prevention learning.
While consecutive cold turkey attempts actually increase the odds of success by inviting self discovery via the school of hard-quitting-knocks of the power of a puff to induce relapse, consecutive NRT attempts actually have the exact opposite effect, in diminishing success rates.
Two studies analyzed success rates among second-time nicotine patch users and the results were devastating. A 1993 study found a 100 percent six-month smoking relapse rate,17 while a 1995 study found that 98.4% of second time patch users failed.18
Closing Thoughts
Quitter deaths are not only the product of direct NRT use failure, but arise from indirect cold turkey quitter demoralization, cold turkey quitter needs neglect, and the destruction of natural lapse/relapse learning.
Sadly, since 1996 U.S. smoking cessation health officials have been sleeping with the pharmaceutical industry, in actually allowing industry consultants to author U.S. quitting policy. In June 2000 they were married. It was then that a stroke of corporate genius resulted in one Guideline cessation policy recommendation consuming all others; that unless medically contraindicated, that all quitters should henceforth purchase and use approved quitting products.19
The policy instantly transformed all public health officials into quitting product salesman and their websites into product storefronts. Instantly, government health officials could no longer offer counseling or support to millions of cold turkey quitters.
And whose stroke of corporate genius was that? I submit that the idea was Glaxo-Wellcome's, as suggested by the purpose clauses of a million dollar University of Wisconsin endowment agreement dated October 15, 1997,20 an agreement whose stated purpose was to "assist the Director of the Center for Tobacco Research and Intervention as indicated." That was a position then held by Dr. Michael Fiore, who would become the primary author of U.S. cessation policy, the June 2000 U.S. Guideline for Treating Tobacco Use and Dependence.
And what possible motivation could Glaxo-Wellcome have had for wanting to "update" the Guidelines so as to create a "new national standard of care" that would require "every patient visiting a healthcare setting" to be "urged to quit and provided with" (among other things) "pharmacotherapy"? On July 1, 1997, with Dr. Fiore's help, Glaxo-Wellcome started marketing Zyban.21
It's beyond time for a divorce. The June 2000 medicinization of smoking cessation brought decline in the U.S. adult smoking rate to a standstill.
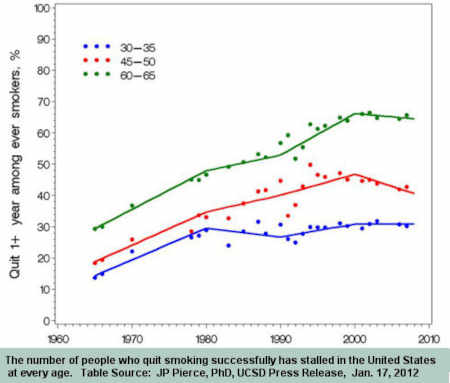
Smoking is America's leading cause of preventable death. Current U.S. cessation policy is contributing to those deaths. It's time for HHS Secretary Kathleen Sebelius to demonstrate understanding and leadership.
It is within Secretary Sebelius' power to immediately suspend current U.S. cessation policy and order HHS to begin servicing the motivation, education, counseling and support needs of America's largest segment of quitters, those trusting their natural instincts.
References:
1 Polito JR, Are those who quit smoking paying with their lives because of NRT's failure? BMJ 2012; 344:e886 BMJ Link
2 Doran CM, Valenti L, Robinson M, Britt H, Mattick RP. Smoking status of Australian general practice patients and their attempts to quit. Addict Behav2006;31:758-66. Medline
3 Pierce JP, Cummins SE, White MM, Humphrey A, Messer K, Quitlines and Nicotine Replacement for Smoking Cessation: Do We Need to Change Policy?, Annu. Rev. Public Health 2012. 33:12.1–12.16. Medline
4 Hartman AM. What does US national population survey data reveal about effectiveness of nicotine replacement therapy on smoking cessation? Paper presented at World Conference on Tobacco or Health, 12-15 July 2006, Washington, DC. FREE Full Text; also see Ferguson J, Bauld L, Chesterman J, Judge K, The English smoking treatment services: one-year outcomes, Addiction. 2005 Apr;100 Suppl 2:59-69. Medline; also see Pierce JP, Gilpin EA, Impact of over-the-counter sales on effectiveness of pharmaceutical aids for smoking cessation, JAMA. 2002 Sept. 11;288(10):1260-4. FREE Full Text
5 Alberg AJ, Patnaik JL, May JW, Hoffman SC, Gitchelle J, Comstock GW, Helzlsouer KJ, Nicotine replacement therapy use among a cohort of smokers. J Addict Dis. 2005;24(1):101-13. Medline
6 Gallup, Most U.S. smokers want to quit, have tried multiple times, July 31, 2013; Ferguson SG, Shiffman S, Gitchell JG, Sembower MA, West R, Unplanned quit attempts--results from a U.S. sample of smokers and ex-smokers, Nicotine Tob Res. 2009 Jul;11(7):827-32. Epub 2009 Jun 9. Medline; also see, West R, Sohal T, "Catastrophic" pathways to smoking cessation: findings from national survey, BMJ. 2006 Feb 25;332(7539):458-60. Epub 2006 Jan 27. Full Text; also see West R, Fidler J (2011) Smoking and Smoking Cessation in England 2010. London: Vasco-Graphics. Unpublished Full Text
8 Borland R, Partos TR, Cummings KM, Systematic Biases in Cross-sectional Community Studies may Underestimate the Effectiveness of Stop-Smoking Medications, Nicotine Tob Res. 2012 Feb 7. Medline
9 Fiore MC, Jaen CR, Baker TB, et al. Treating Tobacco Use and Dependence: 2008 Update. Clinical Practice Guideline. Rockville (MD): U.S. Department of Health and Human Services, Public Health Service, 2008. FREE Full Text
10 Alpert, HR, Connolly GN, Biener, L, A prospective cohort study challenging the effectiveness of population-based medical intervention for smoking cessation, TC Online First, January 10, 2012. Medline
11 Centers for Disease Control and Prevention. Vital Signs: Current Cigarette Smoking Among Adults Aged ≥ 18 Years—United States, 2005 - 2010. Morbidity and Mortality Weekly Report 2011;60(33):1207–12 [accessed 2012 Jan 24]. Vital Signs Link
12 CDC MMWR, Quitting Smoking Among Adults United States, 2001-2010, Weekly November 11, 2011; 60(44);1513-1519. MMWR Link
13 Aubin HJ, Bobak A, Britton JR, Oncken C, Billing CB Jr, Gong J, Williams KE, Reeves KR, Varenicline versus transdermal nicotine patch for smoking cessation: results from a randomised open-label trial, Thorax. 2008 Aug;63(8):717-24. Epub 2008 Feb 8. FREE Full Text
14 Tsukahara H, Noda K, Saku K, A randomized controlled open comparative trial of varenicline vs nicotine patch in adult smokers: efficacy, safety and withdrawal symptoms (the VN-SEESAW study), Circ J. 2010 Apr;74(4):771-8. Epub 2010 Feb 13. FREE Full Text
15 Polito JR, Dying Truths About Quitting Smoking Methods, November 14, 2011, WhyQuit.com
16 Polito JR, SmokeFree.gov Really BuyMeds.now, March 24, 2010, WhyQuit.com
17Tonnesen P, et al, Recycling with nicotine patches in smoking cessation, Addiction, April 1993, Volume 88(4), Page 533-539. Medline
18 Gourlay S. G., et al, Double blind trial of repeated treatment with transdermal nicotine for relapsed smokers, British Medical Journal, 1995 Volume 311, Pages 363–366; see Table III. FREE Full Text
19 Fiore MC et al, Clinical Practice Guideline - Treating Tobacco Use and Dependence, USDHHS , June 2000, Recommendation 7. FREE Full Text
20 University of Wisconsin Foundation Memorandum of Agreement, October 15, 1997. WhyQuit.com
21 Glaxo-Wellcome: PR Newswire, New Aid to Smoking Cessation Treatment Available to Help Smokers Declare Independence from Cigarettes, July 1, 1997. FREE Full Text

