
Nicotine Cessation:
Lost in the Deserts of Abu Dhabi

Host of the 16th World Conference on Tobacco or Health (WCTOH - March 17-21, 2015), the UAE barrenness surrounding attendees won't be as great as the integrity claimed by the synergy of three decades of raw corporate greed, the biases inherent in a battalion of pharma paid researchers, misplaced trust in building academic prestige, and the health community's blind and misguided group-think.
Imagine no page or document on the Conference's website once mentioning "cold turkey," the quitting method this year responsible for generating, in every nation on earth, more ex-smokers and ex-tobacco users than all other methods combined.
Instead, imagine the Conference's official Smoking Cessation "Factsheet", surely reviewed by the Conference's chief science officer, elevating use of "low levels of nicotine" and "lower risk" e-cigarettes above traditional non-medicated abrupt nicotine cessation. Why?
Imagine the marketing genius that rebranded the natural insecticide nicotine "medicine" and labeled its use "therapy." It's been RJ Reynold's long-term vision and business model since at least April 14, 1972. It's a model visibly unfolding before us. Keep your eye on the nicotine.
U.S. cigarette giant Reynolds American, who purchased a nicotine gum company in 2009, moved during 2014 to become a major nicotine gum-maker. India's leading cigarette seller, ITC Limited, started selling Kwiknic gum in 2013. Now, Philip Morris International is about to test market nicotine patch co-inventor Jed Rose's newest smokeless nicotine delivery invention.
Ask yourself, when have we ever once heard any approved quitting product commercial tell smokers why quitting is so important, because smoking kills? And we won't.
And isn't it odd that for nearly a decade Philip Morris USA has been teaching smokers that use of "small amounts of nicotine to help you manage withdrawal symptoms and urges" is key to successful quitting? Or is it?
It's really nothing new. The cigarette industry has been openly operating behind enemy lines since at least 1984.
And why wouldn't they? A July 2013 Gallup Poll found that after 29 years and billions in Nicorette marketing, that only 1 in 100 ex-smokers credit nicotine gum for their success; that all approved products combined account for a tiny fraction of successful cessation (about 8 percent).
How much closer can nicotine gum possibly get to being a complete fraud and totally worthless? How much longer before it's noticed that pharma and tobacco are on the same team, or at least they were until some foxes started buying henhouses?
As shown by the following once secret documents, tobacco control has ignored a 30-year nicotine marketing partnership between the pharmaceutical and tobacco industries (see Bates document # 2023799799, 2023799801, 2023799804, 2023799803, 2023799796, 2023799795, 2023799789, 2500016765, 2083785672, 500872678 and 2064952307).
Will it go unnoticed at the 16th World Conference that at least two presenters at prior conferences, key researchers who helped convince the world of NRT's worth (Saul Shiffman and Jack Henningfield) are now working for America's second largest cigarette maker, Reynolds American?
It's the same Saul Shiffman, PhD who in 2005 partnered with GlaxoSmithKline to invent the need to adjust quitting survey data so as to explain away negative NRT population level findings. NRT's dismal real-world record and the anxieties and frustrations flowing from the failed quitter's inability to successfully wean themselves off nicotine by use of "the best science has to offer," is being used to manufacture a need to make things "fair" via adjustments to raw data.
In deciding to give quitters who self-select NRT an adjustment advantage over non-NRT quitters, Shiffman ignored: (1) that it's impossible to paint an accurate picture of use, quitting and dependency variables when excluding exam of successful quitters, and full smoking, NRT use and quitting histories; (2) the fact that a prior failed NRT attempt highly predisposes failure during a subsequent NRT attempt; and (3) that multiple cessation relapses, many involving prior NRT use, naturally result in longer smoking, tolerance increases, more daily cigarettes, earlier times to first cigarette, and greater quitting difficulty.
It should also be noted that NRT itself has disrupted historical nicotine dependency recovery learning and patterns.
Prior to NRT's 1984 arrival, quitters were able to eventually self-discover via the school of hard-quitting-knocks the power of one hit of nicotine to induce full and complete release, what's known as the Law of Addiction. It's natural learning made muddy or missed when between cold turkey attempts Philip Morris and GlaxoSmithKline are teaching them to reach for nicotine gum medication to experience "intense craving relief."
Thirty Years of Sham Science and Lies
Will the WCTOH discuss the October 2014 finding and long overdue admission of the UK's most conflicted and zealous replacement nicotine (NRT) advocate, Robert West, PhD?
West's prospective study of quitting in England found that "Compared with smokers using none of the cessation aids, the adjusted odds of remaining abstinent up to the time of the 6-month follow-up" ... [after] ... "use of NRT bought over the counter was associated with a lower odds of abstinence (odds ratio, 0.68; 95% CI, 0.49-0.94)."
Further, will anyone question the believability of West's lone remaining finding, that the odds of cessation at "6-month follow-up survey were 2.58 times higher in users of prescription medication"?
Will it be noticed that the use period for nearly all UK quitting prescriptions is 2 to 3 months, while Table 2 of West's study shows that, at 6-month follow-up, up to 71 percent of prescription quitters were still within the first 12 weeks of quitting? If still using "prescription medication," their a4b2-type dopamine pathways receptors were still being chemically stimulated when West declared them quit and free. If so, they'd yet to attempt to adjust to natural stimulation.
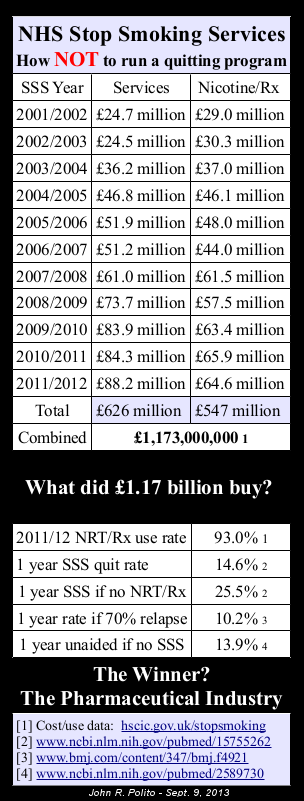 Will any NHS Stop Smoking Services (SSS) WCTOH attendee raise the fact that,
according to national 4-week data (which, due to SSS participants having 2 weeks to begin an attempt more accurately reflects 2-week quitting), not once since 2000 have NRT quitters generated a higher 2-week quitting rate than those quitting without any quitting product?
Will any NHS Stop Smoking Services (SSS) WCTOH attendee raise the fact that,
according to national 4-week data (which, due to SSS participants having 2 weeks to begin an attempt more accurately reflects 2-week quitting), not once since 2000 have NRT quitters generated a higher 2-week quitting rate than those quitting without any quitting product?
Will anyone dare mention the emperor's nakedness, that all independent long-term population level findings over the past decade suggest that OTC NRT (the manner NRT is used by nearly all) is substantially less effective than quitting without it, that it's undercutting successful cessation, and costing lives?
Will there be concern that more agreeable cigarette-like e-cigarettes are riding NRT's "medicine" and "therapy" coat-tails? Extracted from the exact same plant, talk about double standards. How can e-cig industry nicotine be dangerous while pharma's inhaler delivers medicine?
Still, as yet, we have no idea of the percentage of smokers who would have fully arrested their chemical dependence, who instead have been diverted to electronic nicotine, where a worrisome percentage end-up using e-cigarettes to supplement smoking.
Today, nearly every nation's quitting site and literature helps sell nicotine. They paint it as medicine while "lying" about how the vast majority of ex-smokers succeeded (see page 10 of the National Cancer Institute's Clearing the Air).
Tobacco control continues to ignore tobacco's greatest harm of all: life's #1 priority becoming that next nicotine fix; ongoing chemical dependence with mandatory feedings, every waking hour of every day until abated by death or real quitting.
Although harm reduction is a no brainer, try to locate any advocate putting harm elimination first. Good luck.
Frustrated by decades of hidden yet dismal NRT findings, clueless as to the key to successful cold turkey quitting, nicotine cessation is now openly being thrown under the bus. Based on what?
The jury is still out as to whether electronic nicotine is a net health positive or negative. We're guessing at the long-term consequences of inhaling a known lung cancer promoter in animals (nicotine) and countless flavoring chemicals, into lungs seriously compromised by years of smoking.
What we do know is that many e-cig users believe they're now safe. And again, while harm reduction is common sense, should we ignore the fact that successful transfer to electronic nicotine delivery destroys what was previously their greatest justification for fully arresting their addiction, fear of death?
Doesn't common sense dictate that we elevate and put their most powerful motivation to work in helping break nicotine's grip upon their brain, priorities, thinking and life, before advocating and resigning them to increased odds of remaining nicotine's slave for life?
But how can we do that if all we know how to do, if all that's ethical, is handing the addict more nicotine? The current state of quitting? Is it possible to have made a worse mess? Isn't that exactly what the nicotine industry needed and wanted?
Thirty years of "quitting sucks" marketing haven't just helped sell nicotine. It's elevated quitting anxieties. Fear marketing has preyed upon and grossly inflated the up to 72 hours needed to become 100% nicotine-free, sense receptors begin re-sensitizing, and move beyond peak withdrawal.
I often wonder which mind is sickest, the one marketing neurochemical captivity as "freedom," or the mind highly skilled at using assignment awareness frustrations, or the timing and content of counseling and support, to manufacture worthless clinical quitting product "double your chances" efficacy findings.
Intentionally introducing expectations bias by dangling free nicotine in front of nicotine addicts as study recruiting bait, nicotine gum and patch studies resorting to use of active placebos containing just enough nicotine to guarantee serious chronic withdrawal, valuing valueless studies that were impossible to blind as we cannot hide withdrawal's onset from experienced quitters who are experts at recognizing it, the insanity of equating the accomplishment of an addict who weeks ago ended nicotine use to one still using, tailoring study counseling content to favor and promote successful nicotine delivery device transfer while depriving the placebo arm of key info needed to minimize withdrawal, accelerate nicotine elimination and successfully navigate nicotine dependency recovery, it's hard to imagine any study field presenting greater design challenges, or greater opportunity for abuse and fraud, than smoking cessation.
While impossible to determine researcher intent, it certainly doesn't look good when nearly all claiming to be smoking cessation "experts" totally ignore researching the key to success of the method annually generating the vast majority of successful quitters.
Will that question even come up? What is the key to successful cold turkey quitting and why can't it be shared as a quitting tip on a cigarette pack?
At best, countless well-intended researchers, scholars and health policymakers have been totally duped in participating in the greatest research sham in medical history.
Collectively, that research has destroyed nicotine dependency recovery, delayed and frustrated cessation, and during the past 3 decades contributed to millions of premature deaths.
Harry's Hurting Hand
Look no further than the WCTOH's "Welcome Message" co-written by the best of the worst, the 16th World Conference's highly respected "Scientific Programme Committee" Chairman, Harry H. Lando, a University of Minnesota School of Public Health epidemiology professor.
Friendly, heavily used and easily appeased, in fairness, the vast majority of Professor Lando's work has been dedicated to non-NRT research. Still, I fear that his legacy won't be the bulk of his work but his abject failure to protect behavioral cessation science from those who destroyed it.
Professor Lando was an active participant in helping Glaxo Wellcome fulfill its October 15, 1997 written objective, that "every patient visiting a health care setting [be] asked if they use tobacco ... and provided ... pharmacotherapy."
It's worse than simply sleeping with the enemy, worse than, as here, repeatedly serving as cessation's never-rock-the-boat leader. Professor Lando twice co-authored official U.S. cessation policy (Tobacco's June 2000 and May 2008 Clinical Practice Guidelines).
At best, he was twice asleep at the wheel as GlaxoSmithKline's influence assumed total and complete control over U.S. cessation. Paid industry consultants, which included Lando, not only determined which cessation programs the government would thereafter consider "science-based" and support, he helped create ethical roadblocks in government funded research regarding future study of any method which did not include use of approved products.
The actual words? You've already read them. They're nearly identical to the October 15, 1997 delivery promises agreed to by Michael C. Fiore, M.D, the worst of the worst.
Dr. Fiore, the founder and director of the University of Wisconsin's CTRI, has served as pharma's most driven and visible hired gun. He openly sat in a million dollar Glaxo endowed UW chair while he selected and chaired panels which authored what's arguably the deadliest medical policy in history:
June 2000: "7. Numerous effective pharmacotherapies for smoking cessation now exist. Except in the presence of contraindications, these should be used with all patients attempting to quit smoking" (see PDF page 4).
May 2008: "6. Numerous effective medications are available for tobacco dependence, and clinicians should encourage their use by all patients attempting to quit smoking - except when medically contraindicated ..." (see PDF page 7).
Forget the rather glaring concern that neither Fiore nor Lando appear to have appreciated the difference between clinical efficacy and population level effectiveness (as the Guidelines themselves limited studies used to "randomized, placebo/comparison controlled trial[s]").
In totally divorcing common sense, instantly, unassisted abrupt nicotine cessation, the most productive method on earth, had been declared non-science-based. It's mind boggling: use of nicotine is science, while ending use is not.
Pharma's #1 competitor, cold turkey, a method never pitted against NRT, was suddenly black-listed and denied research funding. The giant elephant in the room would henceforth be ignored.
Psychology AWOL
Lando didn't stop there. Worse than helping instantly transform thousands of USDHHS employees into GlaxoSmithKine and Pfizer nicotine, Zyban and Chantix salesmen, with the following words the nicotine industry was handed the quitting turf a Stanford psychology PhD should have had the greatest interest in protecting: behavioral counseling.
"7. Counseling and medication are effective when used by themselves for treating tobacco dependence. The combination of counseling and medication, however, is more effective than either alone. Thus, clinicians should encourage ALL individuals making a quit attempt to use BOTH counseling and medication" (see 2008 Guideline, PDF page 7)
The policy claims to be supported by 2008 Guideline Table 6.24, entitled "Effectiveness of and estimated abstinence rates for the combination of counseling and medication versus counseling alone," a table purporting to be backed by 9 studies.
It's a critical evidence table, as it's pharma influence's means of destroying government backing and support for both stand-alone cognitive behavioral therapy programs (CBT) and cold turkey education, counseling and support programs, such as those at WhyQuit, Joel's Library, and Turkeyville.
Keep in mind that all but one of the following studies (the largest) involved study marketing and/or informed consent followed by randomization, a process which fostered expectations in participants of receiving weeks or months of free nicotine.
As for the specifics of Table 6.24's nine studies: (1) Fagerstrom 1984 is a totally un-replicable nicotine gum study in which 13 physicians conducted 4 follow-up counseling contacts "in their own personal way"; (2) Hall 1985 found that at 52 weeks the difference between intense counseling and intense counseling plus nicotine gum was not significant; (3) Hand 2002 involved 4 weekly counseling sessions and concluded that "in hospital patients NRT, given as regular daily patches plus an inhalator to be used as needed, did not add to the smoking cessation rate achieved at 1 year by regular advice and support ..."; (4) Huber 1988 is a German nicotine gum study with no abstract. According to a 2012 NRT Cochrane Review the behavioral arm involved 5 weekly group meetings, there was no cessation validation and quit rates were derived from graphs; (5) Killen 1984 is a small (20-22 per arm) rapid smoking aversion therapy plus nicotine gum study which notes that "Since there was only one therapist per treatment condition it is possible that therapist characteristics may have accounted for differences in outcome"; (6) Molyneux 2003 is a "brief counseling" study involving either usual care (nothing), a single 20 minute doctor or nurse session at bedside in a hospital with or without randomization to NRT. As for the quality of counseling, the 12 month continuous cessation rate of the usual care/nothing arm was double the rate of counseling only; (7) Ockene 1991 - The study's 1,286 participants may weigh this study heavier than all others combined. That's unfortunate because, according to the study's authors, "The study was not designed to identify the specific impact of the use of nicotine gum." Participants were not randomized to NRT. They individually chose gum use after randomization to either brief quitting advice or brief advice plus 3 monthly 10 minute telephone counseling sessions plus 3 supportive letters; (8) Prapavessis 2006 - CBT counseling actually prevails over CBT + patch (12% vs. 11% point prevalence abstinence at 52 weeks; and (9) Swanson 2003 - Again counseling prevails. Here, at one year follow-up, 180 minutes of upfront quality counseling within the first four weeks, involving the American Cancer Society's Fresh Start program standing alone prevailed (47%) over nicotine patch (20%), bupropion (7%) and patch plus bupropion (27%).
Sadly, there were thousands of abrupt cessation Fresh Start programs in cities across America, prior to being effectively outlawed by the June 2000 Guideline. And it should never be forgotten that GlaxoSmithKline gave millions of dollars in contributions to the American Cancer Society.
Sadly, Professor Lando was willing to sleep with the enemy and hoist the white flag when he should have been fighting hard to protect counseling's right to counsel nicotine-free.
Conflicts, Conflicts and More Conflicts
Since NRT was crowned king, our Nicorette dependent president's administration appointed one of GlaxoSmithKline's finest Nicorette salesman as director of the FDA's new Tobacco Products Office (Mitch Zeller, Esquire).
Also, the CDC's Office on Smoking and Health is today run by the nation's nicotine patch give-a-way champion. Dr. Tim McAfee is a founder of Free & Clear, part of our nation's pharma friendly and NRT dependent telephone quitline (1-800-QUIT-NOW). It's been a 1-800-QUIT-NOW referral beneficiary of the CDC's $50 million per year Tips from Former Smoker's marketing campaign.
"I view the pharmaceutical industry as our ally," said Professor Lando in an interview for a 2007 front-page Wall Street Journal article entitled "Nicotine Fix: Behind Antismoking Policy, Influence of Drug Industry."
But with the industry's dismal six-month OTC NRT quitting rate of 7-8% undercutting unassisted quitting's 10-11% natural rate, and Lando's 1978-79 pre-NRT behavioral programs documenting 75 percent and 76 percent six month rates, why ally yourself with a killer?
Like so many others, the FDA's 1984 approval of Glaxo Wellcome's Nicorette nicotine gum somehow altered Professor Lando's cessation research focus from wanting to help addicts end nicotine use, to successful transfer to new forms of delivery.
Even then, his own 1992 nicotine gum study taught him that incorporating Nicorette into existing community-based quitting programs does not increase long-term success.
Professor Lando also co-authored a nicotine patch study of postmenopausal women published in 2007. There, he found that "Subjects who received transdermal nicotine were significantly more likely than placebo-treated subjects to remain abstinent from smoking during treatment, but not at the 1-year follow-up."
He must have conscious awareness that more than 200 placebo-controlled clinical trials documenting the efficacy of nicotine replacement therapy (NRT) were not blind as claimed, that experienced quitters become experts at recognizing withdrawal's onset, that 3-4 times as many placebo group members are able to correctly declare their assignment as declare wrong, and that they can do so within 24-48 of quitting (peak withdrawal).
But for some reason this friendly scholar's science driven mind doesn't seem to care.
The 2000 Guideline's conflicts disclosure lists Professor Lando as being a consultant to both Glaxo Wellcome and SmithKline Beecham (numbered page 173, PDF page 197).
The 2008 Guideline's disclosure states that "Harry A. Lando reported no significant financial interests. Under additional disclosures, he reported serving on an advisory panel for a new tobacco use cessation medication and attending 2-day meetings in 2005 and 2006 as a member of this panel" (numbered page 226, PDF page 244).
He's also an editor of the journal Addiction. There, his current financial disclosure states: "Harry Lando has received travel funds and has consulted with pharmaceutical companies that manufacture products intended to help smokers to quit. These products include nicotine replacement, Zyban (bupropion), and Chantix (Varenicline). As of May 2006 he no longer accepts compensation or consulting from the pharmaceutical industry."
But his March 2014 CV lists him as being a member of Pfizer Pharmaceutical's National and Regional Advisory Boards and also serving as a consultant to SmithKline Beechem, Dow Chemical Corporation, Vipont Pharmaceuticals, Inc., Key Pharmaceutical, Lederle Pharmaceuticals and Marion Merrell Dow.
Talk about a lack of financial conflicts clarity. It's the same quagmire that's destroyed nicotine cessation. The only remaining question is, will Professor Lando awaken and attempt to right his wrongs while still time? Sadly, I think not.
Co-author of a 2014 study, there he asserts that "Medication includes nicotine replacement therapy (NRT) (patches, gum, sublingual tablets, lozenges, inhalers and nasal spray) and prescribed drugs such as Bupropion and Varenicline. In general, medication is more expensive than medical counseling and quit lines. However, the evidence showed that it can double or triple quit rates."
Money or not, Professor Lando appears unable to stop pushing nicotine as medicine, or grossly overstating its worth.
At what price?
Additional approved product news articles
More cold turkey news articles
WhyQuit News
